J Neurocrit Care.
2023 Dec;16(2):69-76. 10.18700/jnc.230030.
Diurnal variation in quantitative pupillary reactivity in large hemispheric stroke
- Affiliations
-
- 1Department of Neurology, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Critical Care Medicine, Seoul National University Hospital, Seoul, Korea
- 3Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Neurology, Kangdong Sacred Heart Hospital, Seoul, Korea
- KMID: 2549483
- DOI: http://doi.org/10.18700/jnc.230030
Abstract
- Background
Pupillary light reflex (PLR) assessment is an important neurological examination reflecting neurological deterioration in severe stroke cases. This study investigated the impact of diurnal variation in the PLR using quantitative pupillometry in stable patients with large hemispheric stroke.
Methods
We included 35 patients with large hemispheric stroke without neurological worsening, who were admitted to the neurological intensive care unit between April 2017 and November 2021. Quantitative pupillometry was performed every 4 hours. Pupillometer parameters of maximum pupil size, percentage of constriction (%CH), latency (LAT), constriction velocity (CV), dilation velocity (DV), maximum constriction velocity (MCV), and neurological pupil index (NPi) score were recorded. We evaluated changes in the pupillometer parameters over time using linear mixed model analysis.
Results
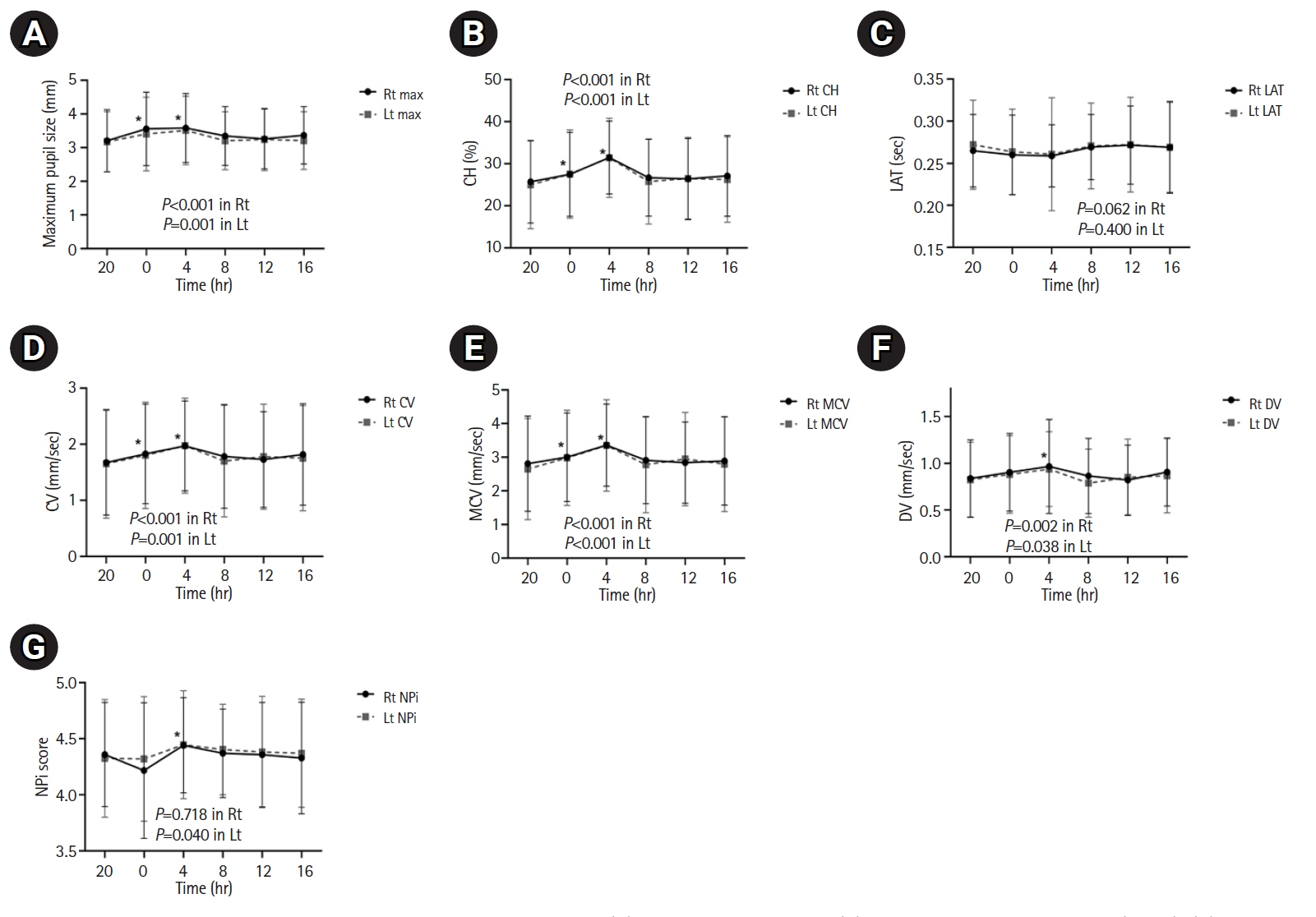
The diurnal variations revealed that the following parameters were significantly higher at 04:00 than at 20:00: maximum pupil size (right [Rt]: 3.59 vs. 3.21 mm, P<0.001; left [Lt]: 3.51 vs. 3.18 mm, P<0.001), %CH (Rt: 31.48 vs. 25.72, P<0.001; Lt: 31.42 vs. 24.98, P<0.001), CV (Rt: 1.97 vs. 1.68 mm/sec, P<0.001; Lt: 1.98 vs. 1.65 mm/sec, P<0.001), and DV (Rt: 0.97 vs. 0.84 mm/sec, P<0.001; Lt: 0.94 vs. 0.82 mm/sec, P=0.001). However, no significant diurnal variations were observed in the NPi values.
Conclusion
Pupillary dynamics based on quantitative pupillometer parameters, including the NPi, demonstrated diurnal variations over 24 hours in large hemispheric stroke patients without neurological worsening. However, all changes in the pupillometer parameters were within normal ranges.
Keyword
Figure
Reference
-
1. Lussier BL, Olson DM, Aiyagari V. Automated pupillometry in neurocritical care: research and practice. Curr Neurol Neurosci Rep. 2019; 19:71.2. Zhao W, Stutzman S, DaiWai O, Saju C, Wilson M, Aiyagari V. Inter-device reliability of the NPi-100 pupillometer. J Clin Neurosci. 2016; 33:79–82.3. Larson MD, Singh V. Portable infrared pupillometry in critical care. Crit Care. 2016; 20:161.4. Kim TJ. Quantitative assessments of pupillary light reflexes in neurocritically ill patients. J Neurocrit Care. 2022; 15:79–87.5. Kim TJ, Park SH, Jeong HB, Ha EJ, Cho WS, Kang HS, et al. Neurological pupil index as an indicator of neurological worsening in large hemispheric strokes. Neurocrit Care. 2020; 33:575–81.6. Chen JW, Gombart ZJ, Rogers S, Gardiner SK, Cecil S, Bullock RM. Pupillary reactivity as an early indicator of increased intracranial pressure: the introduction of the Neurological Pupil index. Surg Neurol Int. 2011; 2:82.7. Oddo M, Sandroni C, Citerio G, Miroz JP, Horn J, Rundgren M, et al. Quantitative versus standard pupillary light reflex for early prognostication in comatose cardiac arrest patients: an international prospective multicenter double-blinded study. Intensive Care Med. 2018; 44:2102–11.8. Daguet I, Bouhassira D, Gronfier C. Baseline pupil diameter is not a reliable biomarker of subjective sleepiness. Front Neurol. 2019; 10:108.9. Wilson MH, Edsell M, Imray C, Wright A; Birmingham Medical Research Expeditionary Society. Changes in pupil dynamics at high altitude: an observational study using a handheld pupillometer. High Alt Med Biol. 2008; 9:319–25.10. Münch M, Léon L, Crippa SV, Kawasaki A. Circadian and wake-dependent effects on the pupil light reflex in response to narrow-bandwidth light pulses. Invest Ophthalmol Vis Sci. 2012; 53:4546–55.11. Wilhelm B, Giedke H, Lüdtke H, Bittner E, Hofmann A, Wilhelm H. Daytime variations in central nervous system activation measured by a pupillographic sleepiness test. J Sleep Res. 2001; 10:1–7.12. Eggert T, Sauter C, Popp R, Zeitlhofer J, Danker-Hopfe H; “Vigilance” of the German Society for Sleep Research and Sleep Medicine (DGSM). The pupillographic sleepiness test in adults: effect of age, gender, and time of day on pupillometric variables. Am J Hum Biol. 2012; 24:820–8.13. Mathôt S. Pupillometry: psychology, physiology, and function. J Cogn. 2018; 1:16.14. Couret D, Boumaza D, Grisotto C, Triglia T, Pellegrini L, Ocquidant P, et al. Reliability of standard pupillometry practice in neurocritical care: an observational, double-blinded study. Crit Care. 2016; 20:99.15. Lee MH, Mitra B, Pui JK, Fitzgerald M. The use and uptake of pupillometers in the intensive care unit. Aust Crit Care. 2018; 31:199–203.16. Lussier BL, Stutzman SE, Atem F, Venkatachalam AM, Perera AC, Barnes A, et al. Distributions and reference ranges for automated pupillometer values in neurocritical care patients. J Neurosci Nurs. 2019; 51:335–40.17. Payne WN, Blair K, Barrett MJ. Anisocoria [Internet]. StatPearls Publishing; 2023 [cited 2023 Oct 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/29261943/.18. Biaggioni I. Circadian clocks, autonomic rhythms, and blood pressure dipping. Hypertension. 2008; 52:797–8.19. Loving RT, Kripke DF, Glazner LK. Circadian rhythms in the human pupil and eyelid. Am J Physiol. 1996; 271(2 Pt 2):R320–4.20. Wilhelm H, Lüdtke H, Wilhelm B. Pupillographic sleepiness testing in hypersomniacs and normals. Graefes Arch Clin Exp Ophthalmol. 1998; 236:725–9.21. Van Egroo M, Gaggioni G, Cespedes-Ortiz C, Ly JQ, Vandewalle G. Steady-state pupil size varies with circadian phase and sleep homeostasis in healthy young men. Clocks Sleep. 2019; 1:240–58.22. Jobanputra AM, Scharf MT, Androulakis IP, Sunderram J. Circadian disruption in critical illness. Front Neurol. 2020; 11:820.23. Telias I, Wilcox ME. Sleep and circadian rhythm in critical illness. Crit Care. 2019; 23:82.24. Lusczek ER, Knauert MP. Light levels in ICU patient rooms: dimming of daytime light in occupied rooms. J Patient Exp. 2021; 8:23743735211033104.25. von Gall C. The effects of light and the circadian system on rhythmic brain function. Int J Mol Sci. 2022; 23:2778.26. Ong C, Hutch M, Smirnakis S. The effect of ambient light conditions on quantitative pupillometry. Neurocrit Care. 2019; 30:316–21.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Quantitative assessments of pupillary light reflexes in neurocritically ill patients
- Diurnal Variation and Sleep Pattern in Depressive Patients
- Seasonal Variation in the Occurrence of Stroke
- Diurnal Variation of Blood Pressure; the Difference between before and after Removal of Pheochromocytoma: Evaluation by Ambulatory Blood Pressure Monitoring
- A Study on the Diurnal Variation of Intraocular Pressure