J Korean Med Sci.
2023 Dec;38(48):e357. 10.3346/jkms.2023.38.e357.
Hepatitis C Virus Seroprevalence in Persons Who Inject Drugs in Korea, 2012–2022: A Multicenter, Retrospective Study
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Department of Psychiatry, Bugok National Hospital, Changnyeong, Korea
- 3Department of Psychiatry, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- 4Department of Psychiatry, Incheon Chamsarang Hospital, Incheon, Korea
- 5Department of Psychiatry, National Forensic Hospital, Gongju, Korea
- 6Department of Psychiatry, Daedong Hospital, Daegu, Korea
- KMID: 2549163
- DOI: http://doi.org/10.3346/jkms.2023.38.e357
Abstract
- Background
Limited data are available on hepatitis C virus (HCV) infection in persons who inject drugs (PWID) in South Korea. The present study aimed to investigate the seroprevalence of HCV antibodies, risk factors for HCV seropositivity, and HCV treatment status in PWID between January 2012 and May 2022.
Methods
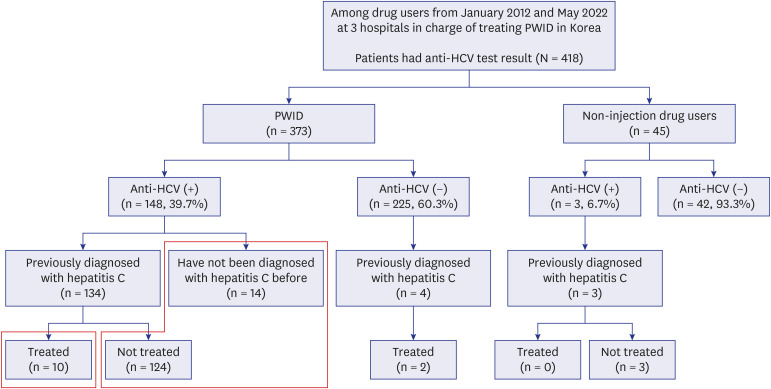
We retrospectively reviewed the medical records of 418 drug users who underwent HCV antibody testing in three hospitals caring for 90% of known PWID in South Korea, of whom 373 were PWID.
Results
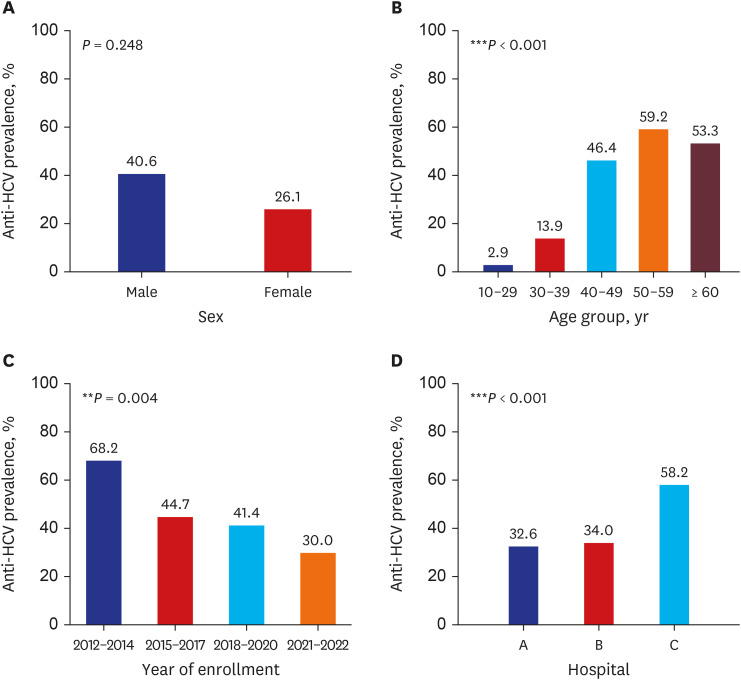
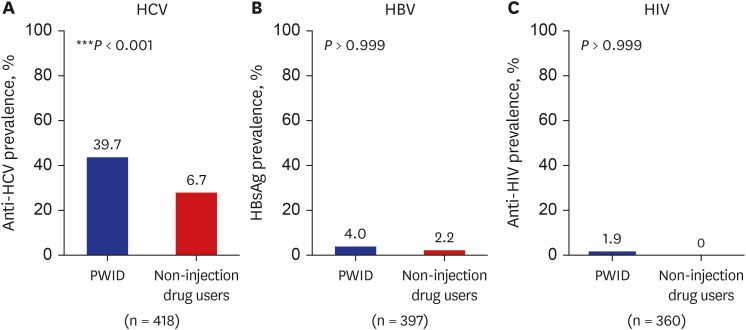
The HCV seroprevalence was 39.7% (148/373) in PWID vs. 6.7% (3/45) in noninjection drug users (P < 0.001). Age ≥ 40 years, hospital type (58.2% in the prison hospital vs. 34.0% in the private hospital), and enrollment year (68.2% in 2012–2014 vs. 30.0% in 2021–2022) were independently associated with HCV seropositivity. Among the HCVseropositive PWID, 90.5% (134/148) were diagnosed with HCV infection; however, only 6.8% (10/148) received HCV treatment. The hepatitis B virus surface antigen and human immunodeficiency virus antibody positivity were 4.0% (14/352) and 1.9% (6/317) in tested PWID, respectively.
Conclusion
The HCV seroprevalence in PWID was 39.7% with a very low treatment rate, which prompts active measures to test and treat PWID for HCV infection in South Korea.
Keyword
Figure
Reference
-
1. Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013; 10(9):553–562. PMID: 23817321.
Article2. World Health Organization. Combating hepatitis B and C to reach elimination by 2030: advocacy brief. Updated 2016. Accessed April 6, 2023. https://apps.who.int/iris/handle/10665/206453 .3. World Health Organization. Interim guidance for country validation of viral hepatitis elimination. Updated 2021. Accessed April 6, 2023. https://apps.who.int/iris/handle/10665/341652 .4. Supreme Prosecutors’ Office of the Republic of Korea. White Paper on Drug-related Crimes. Seoul, Korea: Supreme Prosecutors’ Office of the Republic of Korea;2021.5. Min JA, Yoon Y, Lee HJ, Choi J, Kwon M, Kim K, et al. Prevalence and associated clinical characteristics of hepatitis B, C, and HIV infections among injecting drug users in Korea. J Med Virol. 2013; 85(4):575–582. PMID: 23364858.
Article6. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317–1325. PMID: 16729309.
Article7. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003; 38(2):518–526. PMID: 12883497.
Article8. Trickey A, Fraser H, Lim AG, Peacock A, Colledge S, Walker JG, et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol Hepatol. 2019; 4(6):435–444. PMID: 30981685.
Article9. The Korean Association for the Study of the Liver. 2021 Hepatitis C factsheet. Updated 2021. Accessed April 6, 2023. https://www.kasl.org/bbs/skin/guide/download.php?code=factsheet&number=14469 .10. Jang ES, Ki M, Choi HY, Kim KA, Jeong SH. Korean hepatitis epidemiology study group. The change in the nationwide seroprevalence of hepatitis C virus and the status of linkage to care in South Korea from 2009 to 2015. Hepatol Int. 2019; 13(5):599–608. PMID: 31432446.
Article11. Kim DY, Kim IH, Jeong SH, Cho YK, Lee JH, Jin YJ, et al. A nationwide seroepidemiology of hepatitis C virus infection in South Korea. Liver Int. 2013; 33(4):586–594. PMID: 23356674.
Article12. Kim KA, Lee JS. Prevalence, awareness, and treatment of hepatitis C virus infection in South Korea: evidence from the Korea National Health and Nutrition Examination Survey. Gut Liver. 2020; 14(5):644–651. PMID: 31842525.
Article13. Martin NK, Vickerman P, Dore GJ, Hickman M. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Curr Opin HIV AIDS. 2015; 10(5):374–380. PMID: 26248124.
Article14. Larney S, Kopinski H, Beckwith CG, Zaller ND, Jarlais DD, Hagan H, et al. Incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis. Hepatology. 2013; 58(4):1215–1224. PMID: 23504650.
Article15. Ryan P, Valencia J, Cuevas G, Troya J, Ramon C, Rodríguez A, et al. HCV screening based on dried blood samples and linkage to care in people who use drugs: a prospective study. Int J Drug Policy. 2021; 92:103134. PMID: 33517130.
Article16. Teles SA, Gir E, Martins RM, Dos Santos Carneiro MA, de Matos MA, Caetano KA. Emergent predictors of hepatitis C infection among non-injection drug users. J Infect Public Health. 2018; 11(4):526–529. PMID: 29097105.
Article17. Hermanstyne KA, Bangsberg DR, Hennessey K, Weinbaum C, Hahn JA. The association between use of non-injection drug implements and hepatitis C virus antibody status in homeless and marginally housed persons in San Francisco. J Public Health (Oxf). 2012; 34(3):330–339. PMID: 22451327.
Article18. Nam JY, Jang ES, Kim YS, Lee YJ, Kim IH, Cho SB, et al. Epidemiological and clinical characteristics of hepatitis C virus infection in South Korea from 2007 to 2017: a prospective multicenter cohort study. Gut Liver. 2020; 14(2):207–217. PMID: 31158950.
Article19. Kim KA, Choi GH, Jang ES, Kim YS, Lee YJ, Kim IH, et al. Epidemiology and treatment status of hepatitis C virus infection among people who have ever injected drugs in Korea: a prospective multicenter cohort study from 2007 to 2019 in comparison with non-PWID. Epidemiol Health. 2021; 43:e2021077. PMID: 34645207.
Article20. Graf C, Mücke MM, Dultz G, Peiffer KH, Kubesch A, Ingiliz P, et al. Efficacy of direct-acting antivirals for chronic hepatitis c virus infection in people who inject drugs or receive opioid substitution therapy: a systematic review and meta-analysis. Clin Infect Dis. 2020; 70(11):2355–2365. PMID: 31513710.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevention of Viral Hepatitis and Vaccination
- Seroprevalence of antibody to the hepatitis C virus in methamphetamine abusers
- Seroprevalence of Hepatitis A Virus Among Children and Adults in Phohang Area, Korea
- Changes in the Seroprevalence of Hepatitis A Virus Antibody in Korea
- Ten-Year Trends of Hepatitis A Seroprevalence in People Living with HIV in Korea