Int J Thyroidol.
2023 Nov;16(2):175-183. 10.11106/ijt.2023.16.2.175.
The Role of De novo Serine Biosynthesis from Glucose in Papillary Thyroid Cancer
- Affiliations
-
- 1Research Center for Endocrine and Metabolic Diseases, Chungnam National University College of Medicine, Daejeon, Korea
- 2Departments of Medical Science, Chungnam National University College of Medicine, Daejeon, Korea
- 3Departments of Pathology, Chungnam National University College of Medicine, Daejeon, Korea
- 4Departments of Otolaryngology-Head and Neck Surgery, Chungnam National University College of Medicine, Daejeon, Korea
- 5Departments of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea
- KMID: 2548745
- DOI: http://doi.org/10.11106/ijt.2023.16.2.175
Abstract
- Background and Objectives
The de novo serine biosynthetic pathway from glucose has emerged as one of cancer metabolism; however, it is not explored the interplay between papillary thyroid cancer and metabolic flux of de novo serine synthesis. In this study, we explored the interplay between glucose utilization via GLUT1 expression and phosphoglycerate dehydrogenase (PHGDH).
Materials and Methods
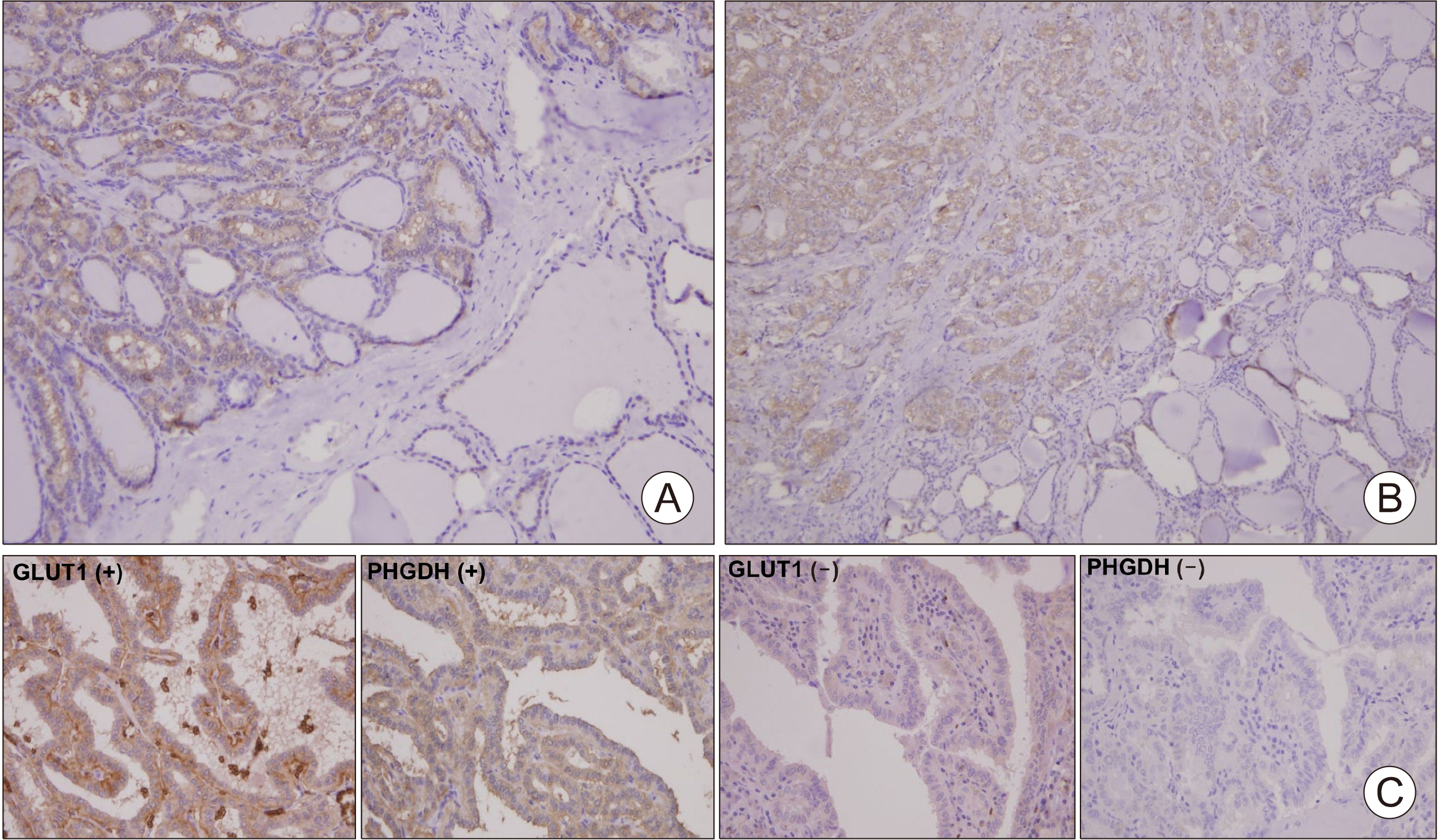
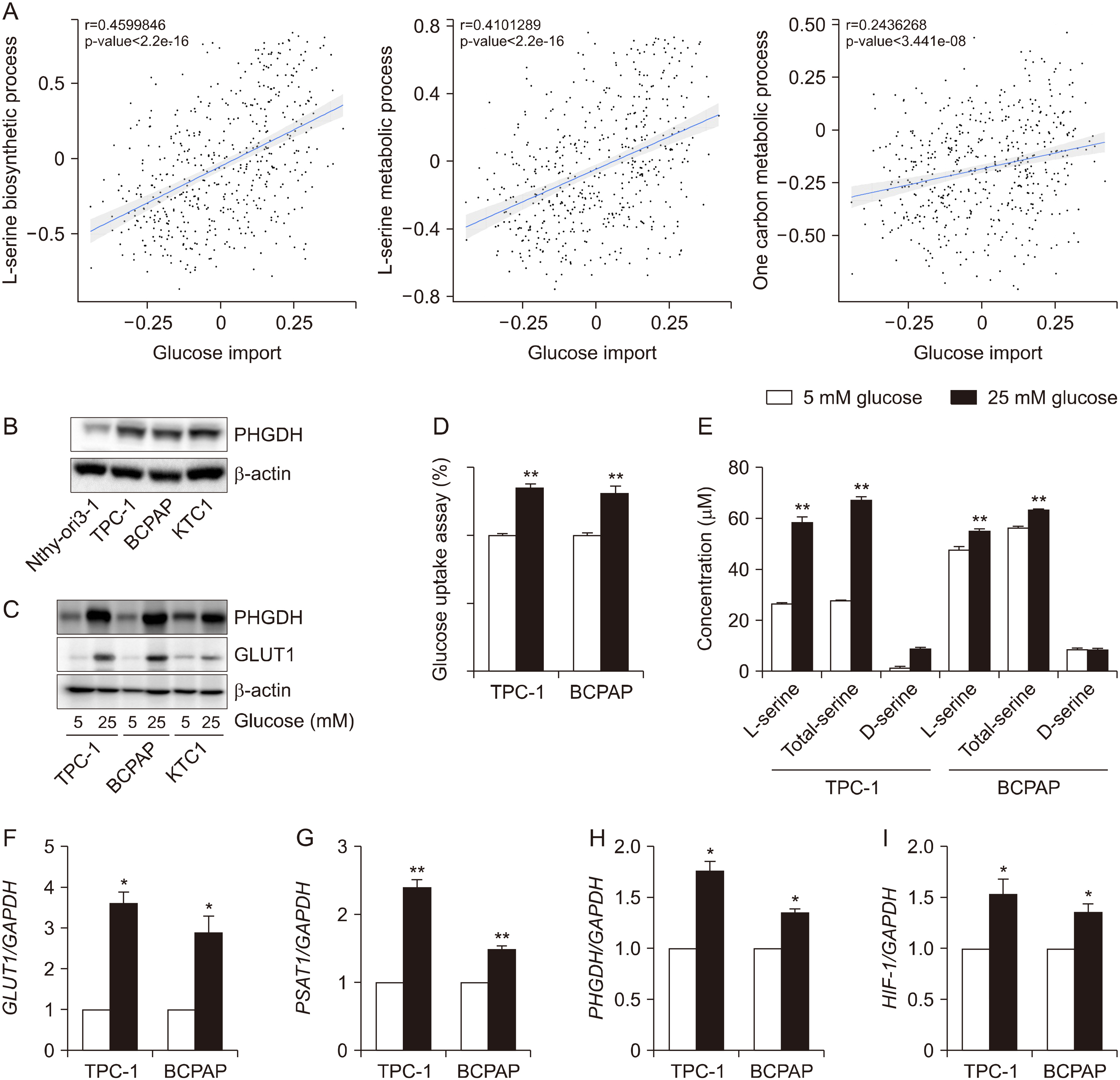
The Cancer Genome Atlas (TCGA) database was used to determine the association between glucose importation and the serine metabolic pathway. The effects of glucose on serine biosynthesis and the role of PHGDH were investigated in papillary thyroid cancer cell lines. PHGDH and GLUT1 expression in 230 patients with papillary thyroid cancer (PTC) was explored using immunohistochemistry to explore the impact of the de novo serine biosynthetic pathway from glucose.
Results
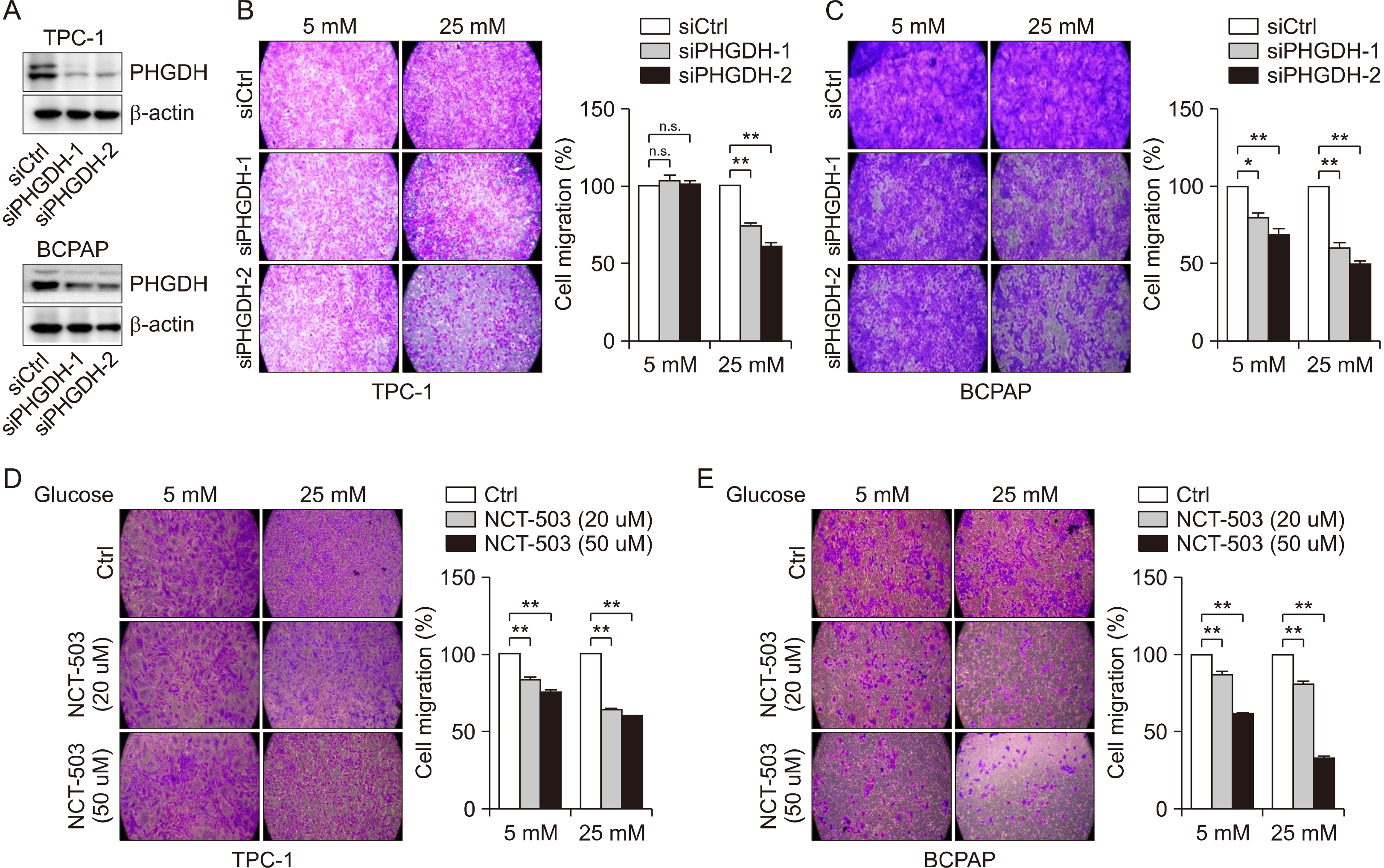
Glucose importation was significantly correlated with the serine biosynthetic and L-serine metabolic processes. Glucose uptake and serine synthesis were significantly increased and mitochondrial complex expression was upregulated in PTC cell lines grown in high-glucose media. Knockdown and inhibition of PHGDH decreased cell migration associated with glucose utilization. High PHGDH expression is significantly related with tumor aggressiveness and GLUT1 expression in patients with PTC.
Conclusion
In this study, we demonstrated that de novo serine biosynthesis from glucose is highly expressed in papillary thyroid cancer and associated with cancer cell metastasis through glucose utility. Our findings suggest the link between glucose utilization PHGDH to regulate tumor aggressiveness in PTC.
Figure
Reference
-
References
1. Kim J, DeBerardinis RJ. 2019; Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab. 30(3):434–46. DOI: 10.1016/j.cmet.2019.08.013. PMID: 31484055. PMCID: PMC6730674.
Article2. Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, et al. 2016; Metabolic heterogeneity in human lung tumors. Cell. 164(4):681–94. DOI: 10.1016/j.cell.2015.12.034. PMID: 26853473. PMCID: PMC4752889.
Article3. Ju SH, Lee SE, Kang YE, Shong M. 2022; Development of metabolic synthetic lethality and its implications for thyroid cancer. Endocrinol Metab (Seoul). 37(1):53–61. DOI: 10.3803/EnM.2022.1402. PMID: 35255601. PMCID: PMC8901971.
Article4. Jeon MJ, You MH, Han JM, Sim S, Yoo HJ, Lee WK, et al. 2020; High phosphoglycerate dehydrogenase expression induces stemness and aggressiveness in thyroid cancer. Thyroid. 30(11):1625–38. DOI: 10.1089/thy.2020.0105. PMID: 32438862. PMCID: PMC7869887.
Article5. Jin M, Lee WK, You MH, Jang A, Cheng SY, Kim WG, et al. 2021; SHMT2 expression as a diagnostic and prognostic marker for thyroid cancer. Endocr Connect. 10(6):630–6. DOI: 10.1530/EC-21-0135. PMID: 34010151. PMCID: PMC8240706.
Article6. Moreno-Sánchez R, Rodríguez-Enríquez S, Marín-Hernández A, Saavedra E. 2007; Energy metabolism in tumor cells. FEBS J. 274(6):1393–418. DOI: 10.1111/j.1742-4658.2007.05686.x. PMID: 17302740.
Article7. Ganapathy-Kanniappan S, Geschwind JF. 2013; Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 12:152. DOI: 10.1186/1476-4598-12-152. PMID: 24298908. PMCID: PMC4223729.
Article8. Oronsky BT, Oronsky N, Fanger GR, Parker CW, Caroen SZ, Lybeck M, et al. 2014; Follow the ATP: tumor energy production: a perspective. Anticancer Agents Med Chem. 14(9):1187–98. DOI: 10.2174/1871520614666140804224637. PMID: 25102360.
Article9. Reina-Campos M, Diaz-Meco MT, Moscat J. 2020; The complexity of the serine glycine one-carbon pathway in cancer. J Cell Biol. 219(1):e201907022. DOI: 10.1083/jcb.201907022. PMID: 31690618. PMCID: PMC7039202.
Article10. Locasale JW. 2013; Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 13(8):572–83. DOI: 10.1038/nrc3557. PMID: 23822983. PMCID: PMC3806315.
Article11. Do SH, Lee SY, Na HS. 2019; The effect of repeated isoflurane exposure on serine synthesis pathway during the developmental period in Caenorhabditis elegans. Neurotoxicology. 71:132–7. DOI: 10.1016/j.neuro.2019.01.001. PMID: 30639121.
Article12. Arlt B, Mastrobuoni G, Wuenschel J, Astrahantseff K, Eggert A, Kempa S, et al. 2021; Inhibiting PHGDH with NCT-503 reroutes glucose-derived carbons into the TCA cycle, independently of its on-target effect. J Enzyme Inhib Med Chem. 36(1):1282–9. DOI: 10.1080/14756366.2021.1935917. PMID: 34192988. PMCID: PMC8253182.
Article13. Martinez-Reyes I, Chandel NS. 2021; Cancer metabolism: looking forward. Nat Rev Cancer. 21(10):669–80. DOI: 10.1038/s41568-021-00378-6. PMID: 34272515.
Article14. Xu X, Peng Q, Jiang X, Tan S, Yang Y, Yang W, et al. 2023; Metabolic reprogramming and epigenetic modifications in cancer: from the impacts and mechanisms to the treatment potential. Exp Mol Med. 55(7):1357–70. DOI: 10.1038/s12276-023-01020-1. PMID: 37394582. PMCID: PMC10394076.
Article15. Liberti MV, Locasale JW. 2016; The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 41(3):211–8. DOI: 10.1016/j.tibs.2015.12.001. PMID: 26778478. PMCID: PMC4783224.
Article16. Abu-Amero KK, Alzahrani AS, Zou M, Shi Y. 2005; High frequency of somatic mitochondrial DNA mutations in human thyroid carcinomas and complex I respiratory defect in thyroid cancer cell lines. Oncogene. 24(8):1455–60. DOI: 10.1038/sj.onc.1208292. PMID: 15608681.
Article17. Su X, Wang W, Ruan G, Liang M, Zheng J, Chen Y, et al. 2016; A comprehensive characterization of mitochondrial genome in papillary thyroid cancer. Int J Mol Sci. 17(10):1594. DOI: 10.3390/ijms17101594. PMID: 27735863. PMCID: PMC5085627.
Article18. Metere A, Graves CE, Chirico M, Caramujo MJ, Pisanu ME, Iorio E. 2020; Metabolomic reprogramming detected by (1)H-NMR spectroscopy in human thyroid cancer tissues. Biology (Basel). 9(6):112. DOI: 10.3390/biology9060112. PMID: 32471147. PMCID: PMC7345942.
Article19. Kurashige T, Shimamura M, Hamada K, Matsuse M, Mitsutake N, Nagayama Y. 2023; Characterization of metabolic reprogramming by metabolomics in the oncocytic thyroid cancer cell line XTC.UC1. Sci Rep. 13(1):149. DOI: 10.1038/s41598-023-27461-2. PMID: 36599897. PMCID: PMC9813134.
Article20. Sun WY, Kim HM, Jung WH, Koo JS. 2016; Expression of serine/glycine metabolism-related proteins is different according to the thyroid cancer subtype. J Transl Med. 14(1):168. DOI: 10.1186/s12967-016-0915-8. PMID: 27277113. PMCID: PMC4898323.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatic Expression of the Serine Palmitoyltransferase Subunit Sptlc2 Reduces Lipid Droplets in the Liver by Activating VLDL Secretion
- Papillary Cancer Arising in Remnant Thyroglossal Duct
- Links between Serine Biosynthesis Pathway and Epigenetics in Cancer Metabolism
- A Case of Papillary Thyroid Carcinoma Arising in a Thyroglossal Duct Cyst with Complete Ectopic Thyroid Gland
- A Case of Ectopic Thyroid Papillary Carcinoma with Incidental Papillary Thyroid Microcarcinoma