J Korean Med Sci.
2023 Nov;38(46):e396. 10.3346/jkms.2023.38.e396.
Comparative Effectiveness of COVID-19 Bivalent Versus Monovalent mRNA Vaccines in the Early Stage of Bivalent Vaccination in Korea: October 2022 to January 2023
- Affiliations
-
- 1Korea Disease Control and Prevention Agency, Cheongju, Korea
- 2Korea University Anam Hospital and Allergy and Immunology Center, Korea University, Seoul, Korea
- KMID: 2548545
- DOI: http://doi.org/10.3346/jkms.2023.38.e396
Abstract
- Background
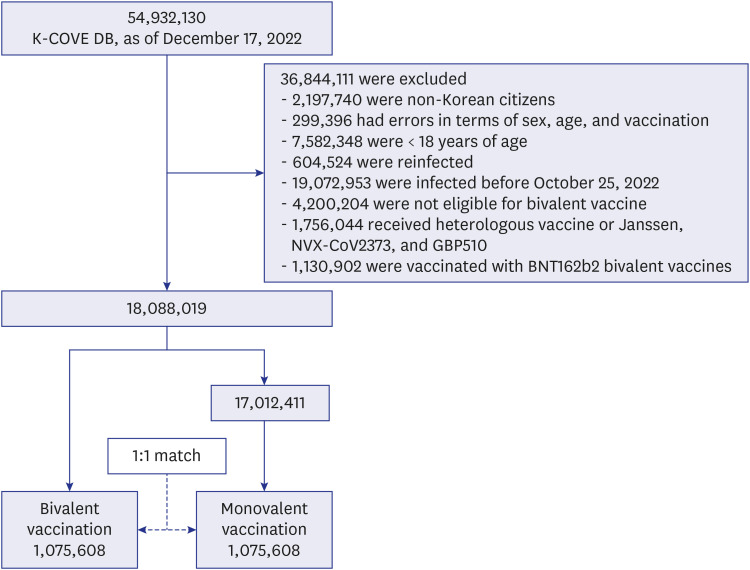
This retrospective observational matched-cohort study of 2,151,216 individuals from the Korean coronavirus disease 2019 (COVID-19) vaccine effectiveness cohort aimed to evaluate the comparative effectiveness of the COVID-19 bivalent versus monovalent vaccines in providing additional protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, critical infection, and death in Korea.
Methods
Among individuals, those vaccinated with COVID-19 bivalent vaccines were matched in a 1:1 ratio with those who were vaccinated with monovalent vaccines (bivalent vaccines non-recipients) during the observation period. We fitted a time-dependent Cox proportional-hazards model to estimate hazard ratios (HRs) of COVID-19 outcomes for infection, critical infection, and death, and we defined vaccine effectiveness (VE) as 1–HR.
Results
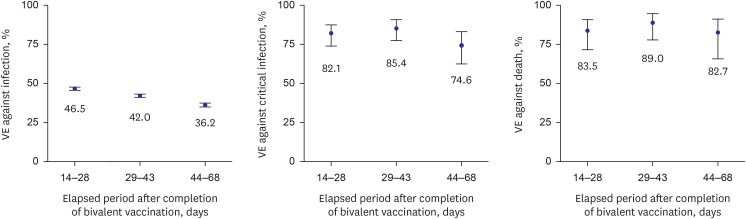
Compared with the bivalent vaccination group, the incidence proportions in the monovalent vaccination group were approximately three times higher for infection, nine times higher for critical infection, and 11 times higher for death. In the early stage of bivalent vaccination, relative VE of bivalent vaccine against monovalent vaccine was 42.4% against SARS-CoV-2 infection, 81.3% against critical infection, and 85.3% against death. In addition, VE against critical infection and death according to the elapsed period after bivalent vaccination was maintained at > 70%.
Conclusion
The bivalent booster dose provided additional protection against SARS-CoV-2 infections, critical infections, and deaths during the omicron variant phase of the COVID-19 pandemic.
Keyword
Figure
Reference
-
1. Onishchenko GG, Sizikova TE, Lebedev VN, Borisevich SV. The Omicron variant of the SARS-CoV-2 virus as the dominant agent of a new risk of disease amid the COVID-19 pandemic. Herald Russ Acad Sci. 2022; 92(4):381–391.
Article2. World Health Organization. One year since the emergence of COVID-19 virus variant Omicron. Updated November 25, 2022. Accessed May 9, 2023. https://www.who.int/news-room/feature-stories/detail/one-year-since-the-emergence-of-omicron .3. Chalkias S, Feng J, Chen X, Zhou H, Marshall JC, Girard B, et al. Neutralization of omicron subvariant BA.2.75 after bivalent vaccination. N Engl J Med. 2022; 387(23):2194–2196. PMID: 36416761.
Article4. Lim S, Sohn M. How to cope with emerging viral diseases: lessons from South Korea’s strategy for COVID-19, and collateral damage to cardiometabolic health. Lancet Reg Health West Pac. 2023; 30:100581. PMID: 36093123.
Article5. Hwang JH, Lee JH, Jang EJ, Kim RK, Lee KH, Park SK, et al. Estimating the number of severe COVID-19 cases and COVID-19-related deaths averted by a nationwide vaccination campaign in Republic of Korea. Osong Public Health Res Perspect. 2023; 14(3):164–172. PMID: 37415433.
Article6. Uraki R, Ito M, Furusawa Y, Yamayoshi S, Iwatsuki-Horimoto K, Adachi E, et al. Humoral immune evasion of the omicron subvariants BQ.1.1 and XBB. Lancet Infect Dis. 2023; 23(1):30–32. PMID: 36495917.
Article7. Ministry of Health and Welfare. Press release: basic directions for the COVID-19 vaccination scheme in the 2022–23 Winter. Updated August 31, 2022. Accessed May 9, 2023. https://www.mohw.go.kr/board.es?mid=a20401000000&bid=0032 .8. Yoo KJ, Kwon S, Choi Y, Bishai DM. Systematic assessment of South Korea’s capabilities to control COVID-19. Health Policy. 2021; 125(5):568–576. PMID: 33692005.9. Jang EJ, Choe YJ, Kim RK, Park YJ. BNT162b2 Vaccine effectiveness against the SARS-CoV-2 Omicron variant in children aged 5 to 11 years. JAMA Pediatr. 2023; 177(3):319–320. PMID: 36622683.10. Kim YY, Choe YJ, Kim J, Kim RK, Jang EJ, Lee H, et al. Vaccine effectiveness against severe disease and death for patients with COVID-19 during the delta-dominant and omicron-emerging periods: a K-COVE study. J Korean Med Sci. 2023; 38(11):e87. PMID: 36942395.
Article11. Kim J, Choe YJ, Jang EJ, Lim DS, Kim YY, Kim RK, et al. Effectiveness of booster mRNA vaccines against SARS-CoV-2 infection in an elderly population, South Korea, October 2021–January 2022. Clin Infect Dis. 2022; 75(5):920–921. PMID: 35439294.
Article12. Kim YY, Choe YJ, Kim J, Kim RK, Jang EJ, Park SK, et al. Effectiveness of second mRNA COVID-19 booster vaccine in immunocompromised persons and long-term care facility residents. Emerg Infect Dis. 2022; 28(11):2165–2170. PMID: 36191615.
Article13. Korea Disease Control and Prevention Agency. COVID-19 vaccination. Updated 2023. Accessed May 9, 2023. https://ncv.kdca.go.kr/eng/ .14. Yi S, Choe YJ, Lim DS, Lee HR, Kim J, Kim YY, et al. Impact of national COVID-19 vaccination campaign, South Korea. Vaccine. 2022; 40(26):3670–3675. PMID: 35570077.
Article15. Arbel R, Peretz A, Sergienko R, Friger M, Beckenstein T, Yaron S, et al. Effectiveness of the bivalent mRNA vaccine in preventing severe COVID-19 outcomes: an observational cohort study. Lancet. 2023.
Article16. Link-Gelles R, Ciesla AA, Fleming-Dutra KE, Smith ZR, Britton A, Wiegand RE, et al. Effectiveness of bivalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection— increasing community access to testing program, United States, September–November 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(48):1526–1530. PMID: 36454688.
Article17. Bobrovitz N, Ware H, Ma X, Li Z, Hosseini R, Cao C, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023; 23(5):556–567. PMID: 36681084.
Article18. Shi HJ, Yang J, Eom JS, Ko JH, Peck KR, Kim UJ, et al. Clinical characteristics and risk factors for mortality in critical COVID-19 patients aged 50 years or younger during Omicron wave in Korea: comparison with patients older than 50 years of age. J Korean Med Sci. 2023; 38(28):e217. PMID: 37463688.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- COVID-19 Vaccination in Korea
- Low Neutralizing Activities to the Omicron Subvariants BN.1 and XBB.1.5 of Sera From the Individuals Vaccinated With a BA.4/5-Containing Bivalent mRNA Vaccine
- Effectiveness of Bivalent mRNA Booster Vaccine Against COVID-19 in Korea
- The incidence and clinical characteristics of myocarditis and pericarditis following mRNA-based COVID-19 vaccination in Republic of Korea adolescents from July 2021 to September 2022
- Cutaneous Adverse Reaction After COVID-19 Vaccination