Restor Dent Endod.
2023 May;48(2):e14. 10.5395/rde.2023.48.e14.
Effect of an aluminum chloride hemostatic agent on the dentin shear bond strength of a universal adhesive
- Affiliations
-
- 1Department of Conservative Dentistry, Wonkwang University, School of Dentistry, Iksan, Korea
- KMID: 2548217
- DOI: http://doi.org/10.5395/rde.2023.48.e14
Abstract
Objectives
This study investigated the effect of an aluminum chloride hemostatic agent on the shear bond strength (SBS) of a universal adhesive to dentin.
Materials and Methods
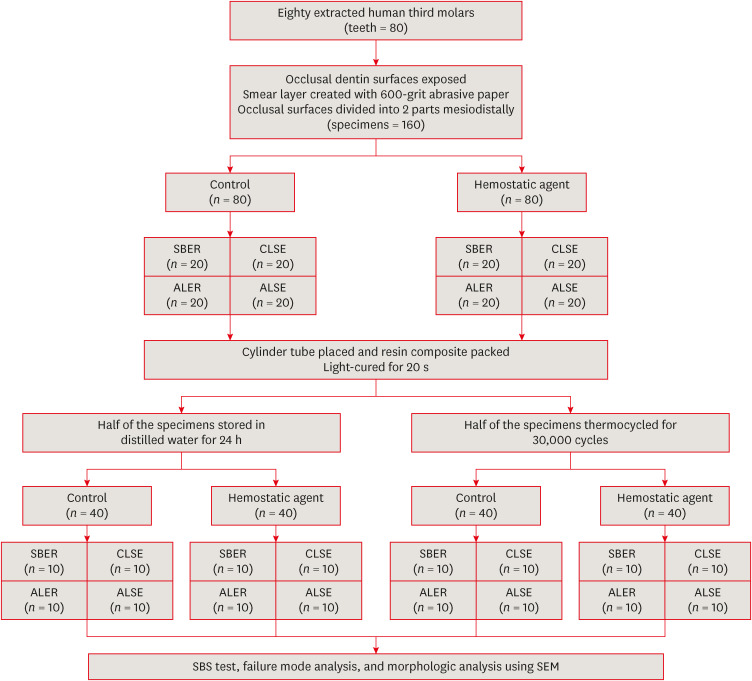
Eighty extracted human molars were trimmed at the occlusal dentin surfaces and divided mesiodistally. According to hemostatic agent application, specimens were randomly allocated into control (C) and hemostatic agent (Traxodent; H) groups. Each group was divided into 4 subgroups according to the adhesive system (n = 20): Scotchbond Multi-Purpose (SBER), Clearfil SE Bond (CLSE), All-Bond Universal etch-and-rinse mode (ALER), and All-Bond Universal self-etch mode (ALSE). SBS was measured for half of the specimens at 24 hours, and the other half were thermocycled in water baths (group T). Fracture surfaces were examined to determine the failure mode. The SBS was measured, and data were analyzed using 1-way analysis of variance, the Student’s t-test, and the Tukey honestly significant difference test (p = 0.05).
Results
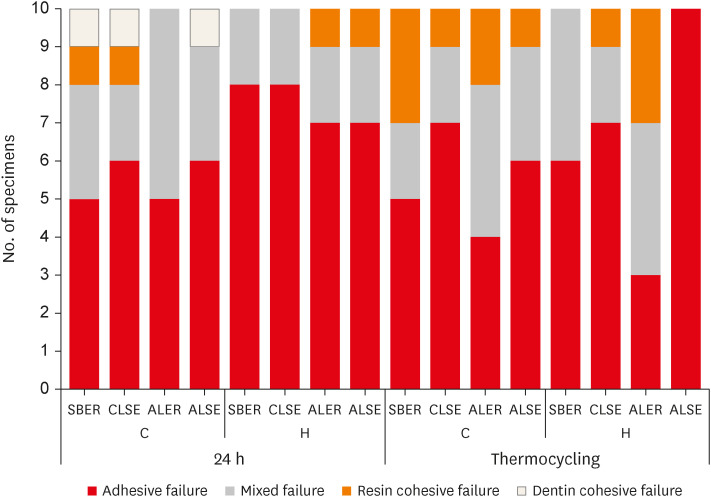
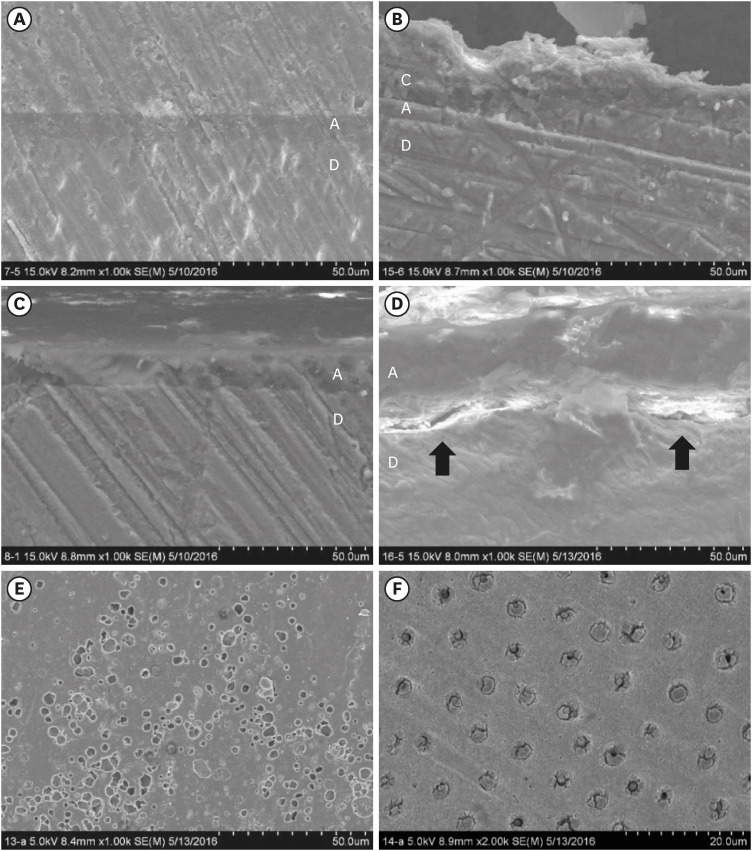
No significant differences in SBS were found between groups C and H for any adhesive system at 24 hours. After thermocycling, a statistically significant difference was observed between CT+ALSE and HT+ALSE (p < 0.05). When All-Bond Universal was applied to hemostatic agent-contaminated dentin, the SBS of H+ALSE was significantly lower than that of H+ALER (p< 0.05). The SBER subgroups showed no significant differences in SBS regardless of treatment and thermocycling.
Conclusions
When exposed dentin was contaminated by an aluminum chloride hemostatic agent before dentin adhesive treatment, application of All-Bond Universal in etch-and-rinse mode was superior to self-etch mode.
Keyword
Figure
Reference
-
1. Yoo HM, Pereira PN. Effect of blood contamination with 1-step self-etching adhesives on microtensile bond strength to dentin. Oper Dent. 2006; 31:660–665. PMID: 17153973.
Article2. Yoo HM, Oh TS, Pereira PN. Effect of saliva contamination on the microshear bond strength of one-step self-etching adhesive systems to dentin. Oper Dent. 2006; 31:127–134. PMID: 16536204.
Article3. de Carvalho Mendonça EC, Vieira SN, Kawaguchi FA, Powers J, Matos AB. Influence of blood contamination on bond strength of a self-etching system. Eur J Dent. 2010; 4:280–286. PMID: 20613916.
Article4. Tarighi P, Khoroushi M. A review on common chemical hemostatic agents in restorative dentistry. Dent Res J. 2014; 11:423–428.5. Ajami AA, Kahnamoii MA, Kimyai S, Oskoee SS, Pournaghi-Azar F, Bahari M, Firouzmandi M. Effect of three different contamination removal methods on bond strength of a self-etching adhesive to dentin contaminated with an aluminum chloride hemostatic agent. J Contemp Dent Pract. 2013; 14:26–33. PMID: 23579888.
Article6. Kuphasuk W, Harnirattisai C, Senawongse P, Tagami J. Bond strengths of two adhesive systems to dentin contaminated with a hemostatic agent. Oper Dent. 2007; 32:399–405. PMID: 17695614.
Article7. Land MF, Couri CC, Johnston WM. Smear layer instability caused by hemostatic agents. J Prosthet Dent. 1996; 76:477–482. PMID: 8933436.
Article8. Chaiyabutr Y, Kois JC. The effect of tooth-preparation cleansing protocol on the bond strength of self-adhesive resin cement to dentin contaminated with a hemostatic agent. Oper Dent. 2011; 36:18–26. PMID: 21488725.
Article9. Land MF, Rosenstiel SF, Sandrik JL. Disturbance of the dentinal smear layer by acidic hemostatic agents. J Prosthet Dent. 1994; 72:4–7. PMID: 8083841.
Article10. Shadman N, Farzin Ebrahimi S, Mollaie N. Sealing of adhesive systems in ferric sulfate-contaminated dentinal margins in class V composite resin restorations. J Dent Res Dent Clin Dent Prospect. 2016; 10:17–22.
Article11. Sharafeddin F, Farhadpour H. Evaluation of Shear Bond Strength of Total- and Self-etching Adhesive Systems after Application of Chlorhexidine to Dentin Contaminated with a Hemostatic Agent. J Dent (Shiraz). 2015; 16:175–181. PMID: 26331146.12. Baba NZ, Goodacre CJ, Jekki R, Won J. Gingival displacement for impression making in fixed prosthodontics: contemporary principles, materials, and techniques. Dent Clin North Am. 2014; 58:45–68. PMID: 24286645.13. Adamis Z, Williams RB. International Programme on Chemical Safety. Environmental Health Criteria 231. Bentonite, kaolin and selected clay minerals. Geneva: World Health Organization;2005.14. Kheirabadi BS, Mace JE, Terrazas IB, Fedyk CG, Estep JS, Dubick MA, Blackbourne LH. Safety evaluation of new hemostatic agents, smectite granules, and kaolin-coated gauze in a vascular injury wound model in swine. J Trauma. 2010; 68:269–278. PMID: 20154537.
Article15. Pourshahrestani S, Zeimaran E, Djordjevic I, Kadri NA, Towler MR. Inorganic hemostats: the state-of-the-art and recent advances. Mater Sci Eng C. 2016; 58:1255–1268.
Article16. Harnirattisai C, Kuphasuk W, Senawongse P, Tagami J. Bond strengths of resin cements to astringent-contaminated dentin. Oper Dent. 2009; 34:415–422. PMID: 19678446.
Article17. Tuncer D, Başaran S, Halaçoglu DM, Yamanel K, Çelik Ç, Arhun N. Effect of haemostatic agent application on the shear bond strength of contemporary/multi-mode adhesive systems. Oral Health Dent Manag. 2014; 13:103–106. PMID: 24603925.18. Chaiyabutr Y, Kois JC. The effects of tooth preparation cleansing protocols on the bond strength of self-adhesive resin luting cement to contaminated dentin. Oper Dent. 2008; 33:556–563. PMID: 18833862.
Article19. Acar Ö, Erkut S, Özçelik TB, Ozdemır E, Akçil M. A clinical comparison of cordless and conventional displacement systems regarding clinical performance and impression quality. J Prosthet Dent. 2014; 111:388–394. PMID: 24360008.
Article20. Ayo-Yusuf OA, Driessen CH, Botha AJ. SEM-EDX study of prepared human dentine surfaces exposed to gingival retraction fluids. J Dent. 2005; 33:731–739. PMID: 16199281.
Article21. Höök M, Christoffersen J, Christoffersen MR, Leonardsen ES, Rassing MR, Rostrup E. Effects of aluminum (III) and fluoride on the demineralization of bovine enamel: a longitudinal microradiographic study. Scand J Dent Res. 1994; 102:198–201. PMID: 8091118.
Article22. Bernades KO, Hilgert LA, Ribeiro AP, Garcia FC, Pereira PN. The influence of hemostatic agents on dentin and enamel surfaces and dental bonding: a systematic review. J Am Dent Assoc. 2014; 145:1120–1128. PMID: 25359643.23. Gale MS, Darvell BW. Thermal cycling procedures for laboratory testing of dental restorations. J Dent. 1999; 27:89–99. PMID: 10071465.
Article24. Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, Inoue S, Tagawa Y, Suzuki K, De Munck J, Van Meerbeek B. Comparative study on adhesive performance of functional monomers. J Dent Res. 2004; 83:454–458. PMID: 15153451.
Article25. Tay FR, King NM, Chan KM, Pashley DH. How can nanoleakage occur in self-etching adhesive systems that demineralize and infiltrate simultaneously? J Adhes Dent. 2002; 4:255–269. PMID: 12666745.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Difference in Bonding Strength of RMGIC according to Type of Hemostatic Agent in Primary Tooth
- Effect of Aluminum Chloride Hemostatic Agent on Bonding Strength of RMGIC in Primary Tooth
- The effect of saliva decontamination procedures on dentin bond strength after universal adhesive curing
- The influence of hemostatic agent contamination on bond strengths on dentin bonding agents
- Effect of applying adhesive after enamel etching on the shear bond strength of orthodontic brackets using light curing resin cements