Restor Dent Endod.
2023 Feb;48(1):e6. 10.5395/rde.2023.48.e6.
High-plasticity mineral trioxide aggregate and its effects on M1 and M2 macrophage viability and adherence, phagocyte activity, production of reactive oxygen species, and cytokines
- Affiliations
-
- 1Department of Restorative Dentistry, Faculty of Dentistry, Federal University of Minas Gerais (UFMG), Belo Horizonte, MG, Brazil
- KMID: 2548174
- DOI: http://doi.org/10.5395/rde.2023.48.e6
Abstract
Objectives
This study evaluated the effects of high-plasticity mineral trioxide aggregate (MTA-HP) on the activity of M1 and M2 macrophages, compared to white MTA (Angelus).
Materials and Methods
Peritoneal inflammatory M1 (from C57BL/6 mice) and M2 (from BALB/c mice) macrophages were cultured in the presence of the tested materials. Cell viability (MTT and trypan blue assays), adhesion, phagocytosis, reactive oxygen species (ROS) production, and tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β production were evaluated. Parametric analysis of variance and the non-parametric Kruskal-Wallis test were used. Results were considered significant when p < 0.05.
Results
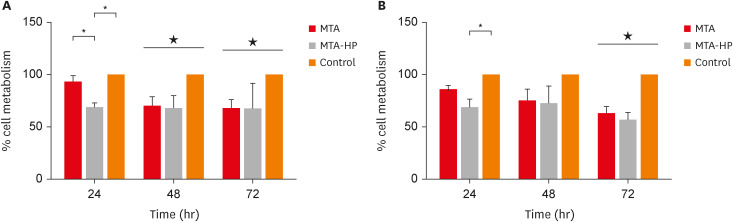
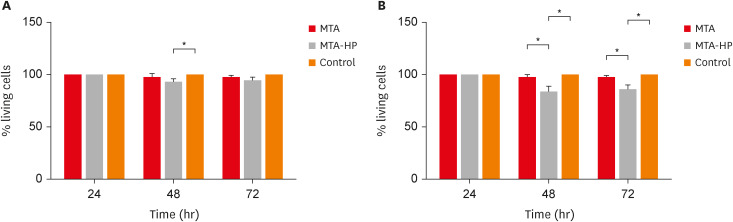
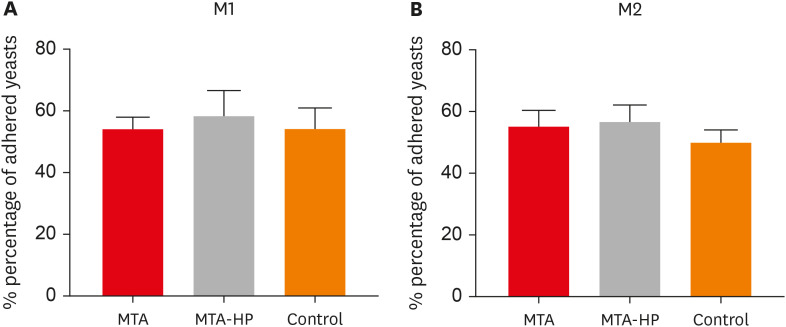
The MTT assay revealed a significant decrease in M1 metabolism with MTA-HP at 24 hours, and with MTA and MTA-HP later. The trypan blue assay showed significantly fewer live M1 at 48 hours and live M2 at 48 and 72 hours with MTA-HP, compared to MTA. M1 and M2 adherence and phagocytosis showed no significant differences compared to control for both materials. Zymosan A stimulated ROS production by macrophages. In the absence of interferon-γ, TNF-α production by M1 did not significantly differ between groups. For M2, both materials showed higher TNF-α production in the presence of the stimulus, but without significant between-group differences. Likewise, TGF-β production by M1 and M2 macrophages was not significantly different between the groups.
Conclusions
M1 and M2 macrophages presented different viability in response to MTA and MTA-HP at different time points. Introducing a plasticizer into the MTA vehicle did not interfere with the activity of M1 and M2 macrophages.
Keyword
Figure
Reference
-
1. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--Part I: chemical, physical, and antibacterial properties. J Endod. 2010; 36:16–27. PMID: 20003930.
Article2. Hench LL. Bioceramics: from concept to clinic. J Am Ceram Soc. 1991; 74:1487–1510.
Article3. Reyes-Carmona JF, Felippe MS, Felippe WT. Biomineralization ability and interaction of mineral trioxide aggregate and white Portland cement with dentin in a phosphate-containing fluid. J Endod. 2009; 35:731–736. PMID: 19410094.
Article4. Emara R, Elhennawy K, Schwendicke F. Effects of calcium silicate cements on dental pulp cells: a systematic review. J Dent. 2018; 77:18–36. PMID: 30086349.
Article5. Felippe WT, Felippe MC, Rocha MJ. The effect of mineral trioxide aggregate on the apexification and periapical healing of teeth with incomplete root formation. Int Endod J. 2006; 39:2–9. PMID: 16409322.
Article6. Scarparo RK, Haddad D, Acasigua GA, Fossati AC, Fachin EV, Grecca FS. Mineral trioxide aggregate-based sealer: analysis of tissue reactions to a new endodontic material. J Endod. 2010; 36:1174–1178. PMID: 20630293.
Article7. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999; 25:197–205. PMID: 10321187.
Article8. Guimarães BM, Prati C, Duarte MA, Bramante CM, Gandolfi MG. Physicochemical properties of calcium silicate-based formulations MTA Repair HP and MTA Vitalcem. J Appl Oral Sci. 2018; 26:e2017115. PMID: 29641748.
Article9. Jiménez-Sánchez MC, Segura-Egea JJ, Diaz-Cuenca A. Physicochemical parameters-hydration performance relationship of the new endodontic cement MTA Repair HP. J Clin Exp Dent. 2019; 11:739–744.10. Palczewska-Komsa M, Kaczor-Wiankowska K, Nowicka A. New bioactive calcium silicate cement mineral trioxide aggregate repair high plasticity (MTA HP)-a systematic review. Materials (Basel). 2021; 14:4573. PMID: 34443098.
Article11. Escobar-García DM, Medina-Rosas MG, González-Amaro AM, Méndez-González V, Flores H, Pozos-Guillén A. MTA-based cements: biocompatibility and effects on the gene expression of collagen type 1 and TGF-β1. BioMed Res Int. 2022; 2022:2204698. PMID: 35402617.
Article12. Braga JM, Oliveira RR, Martins RC, Ribeiro Sobrinho AP. The effects of a mineral trioxide aggregate-based sealer on the production of reactive oxygen species, nitrogen species and cytokines by two macrophage subtypes. Int Endod J. 2014; 47:909–919. PMID: 24354338.
Article13. de Oliveira Mendes ST, Ribeiro Sobrinho AP, de Carvalho AT, de Souza Côrtes MI, Vieira LQ. In vitro evaluation of the cytotoxicity of two root canal sealers on macrophage activity. J Endod. 2003; 29:95–99. PMID: 12597705.
Article14. Rezende TM, Vargas DL, Cardoso FP, Sobrinho AP, Vieira LQ. Effect of mineral trioxide aggregate on cytokine production by peritoneal macrophages. Int Endod J. 2005; 38:896–903. PMID: 16343117.
Article15. Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005; 23:901–944. PMID: 15771589.
Article16. Bastos KR, Alvarez JM, Marinho CR, Rizzo LV, Lima MR. Macrophages from IL-12p40-deficient mice have a bias toward the M2 activation profile. J Leukoc Biol. 2002; 71:271–278. PMID: 11818448.
Article17. Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009; 86:1065–1073. PMID: 19741157.
Article18. Rezende TM, Vieira LQ, Cardoso FP, Oliveira RR, de Oliveira Mendes ST, Jorge ML, Ribeiro Sobrinho AP. The effect of mineral trioxide aggregate on phagocytic activity and production of reactive oxygen, nitrogen species and arginase activity by M1 and M2 macrophages. Int Endod J. 2007; 40:603–611. PMID: 17627697.
Article19. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008; 8:958–969. PMID: 19029990.
Article20. Abou ElReash A, Hamama H, Grawish M, Saeed M, Zaen El-Din AM, Shahin MA, Zhenhuan W, Xiaoli X. A laboratory study to test the responses of human dental pulp stem cells to extracts from three dental pulp capping biomaterials. Int Endod J. 2021; 54:1118–1128. PMID: 33567103.
Article21. Cintra LT, Benetti F, de Azevedo Queiroz ÍO, de Araújo Lopes JM, Penha de Oliveira SH, Sivieri Araújo G, Gomes-Filho JE. Cytotoxicity, biocompatibility, and biomineralization of the new high-plasticity MTA material. J Endod. 2017; 43:774–778. PMID: 28320539.
Article22. Barczak K, Palczewska-Komsa M, Lipski M, Chlubek D, Buczkowska-Radlińska J, Baranowska-Bosiacka I. The influence of new silicate cement mineral trioxide aggregate (MTA Repair HP) on metalloproteinase MMP-2 and MMP-9 expression in cultured THP-1 macrophages. Int J Mol Sci. 2020; 22:295. PMID: 33396675.
Article23. Braga JM, Oliveira RR, de Castro Martins R, Vieira LQ, Sobrinho AP. Assessment of the cytotoxicity of a mineral trioxide aggregate-based sealer with respect to macrophage activity. Dent Traumatol. 2015; 31:390–395. PMID: 26086068.
Article24. Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993; 54:283–288. PMID: 8409750.
Article25. Giaimis J, Lombard Y, Makaya-Kumba M, Fonteneau P, Poindron P. A new and simple method for studying the binding and ingestion steps in the phagocytosis of yeasts. J Immunol Methods. 1992; 154:185–193. PMID: 1383342.
Article26. Trusk MA, Wilson ME, Dyke KV. The generation of chemiluminescence by phagocytic cells. Methods Enzymol. 1978; 57:462–493.27. Mishra P, Singh U, Pandey CM, Mishra P, Pandey G. Application of student’s t-test, analysis of variance, and covariance. Ann Card Anaesth. 2019; 22:407–411. PMID: 31621677.
Article28. Hazra A, Gogtay N. Biostatistics series module 3: comparing groups: numerical variables. Indian J Dermatol. 2016; 61:251–260. PMID: 27293244.
Article29. Ferreira CM, Sassone LM, Gonçalves AS, de Carvalho JJ, Tomás-Catalá CJ, García-Bernal D, Oñate-Sánchez RE, Rodríguez-Lozano FJ, Silva EJ. Physicochemical, cytotoxicity and in vivo biocompatibility of a high-plasticity calcium-silicate based material. Sci Rep. 2019; 9:3933. PMID: 30850648.30. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005; 4:281–286. PMID: 16101534.
Article31. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000; 164:6166–6173. PMID: 10843666.
Article32. Collado-González M, López-García S, García-Bernal D, Oñate-Sánchez RE, Tomás-Catalá CJ, Moraleda JM, Lozano A, Forner L, Rodríguez-Lozano FJ. Biological effects of acid-eroded MTA Repair HP and ProRoot MTA on human periodontal ligament stem cells. Clin Oral Investig. 2019; 23:3915–3924.
Article33. Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans. 2001; 29:345–349. PMID: 11356180.
Article34. Xanthoulea S, Pasparakis M, Kousteni S, Brakebusch C, Wallach D, Bauer J, Lassmann H, Kollias G. Tumor necrosis factor (TNF) receptor shedding controls thresholds of innate immune activation that balance opposing TNF functions in infectious and inflammatory diseases. J Exp Med. 2004; 200:367–376. PMID: 15289505.
Article35. Maciel KF, Neves de Brito LC, Tavares WL, Moreira G, Nicoli JR, Vieira LQ, Ribeiro Sobrinho AP. Cytokine expression in response to root canal infection in gnotobiotic mice. Int Endod J. 2012; 45:354–362. PMID: 22233143.
Article36. Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998; 16:137–161. PMID: 9597127.37. Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-β - an excellent servant but a bad master. J Transl Med. 2012; 10:183. PMID: 22943793.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rhamnazin inhibits LPS-induced inflammation and ROS/RNS in raw macrophages
- Effects of Particulate Matters on A549 and RAW 264.7 Cells
- Effect of Dipyridamole on the Reactive Oxygen Species and Oxidative Stress in Trabecular Meshwork Cells
- Induction of IL-8 and reactive oxygen species in periodontal ligament cells by Aggregatibacter actinomycetemcomitans
- Effect of Erythromycin on Pro-inflammatory Signalings by Particles