Diabetes Metab J.
2023 Nov;47(6):771-783. 10.4093/dmj.2022.0265.
Effects Of Exercise Training And Chlorogenic Acid Supplementation On Hepatic Lipid Metabolism In Prediabetes Mice
- Affiliations
-
- 1Department of Exercise Physiology, Faculty of Sport Sciences, University of Isfahan, Isfahan, Iran
- 2Department of Reproductive Biotechnology, Reproductive Biomedicine Research Center, ACECR, Royan Institute for Biotechnology, Isfahan, Iran
- 3Department of Sports Science, Seoul National University of Science and Technology, Seoul, Korea
- 4Sports Science Research Institute, Seoul National University of Science and Technology, Seoul, Korea
- KMID: 2548154
- DOI: http://doi.org/10.4093/dmj.2022.0265
Abstract
- Background
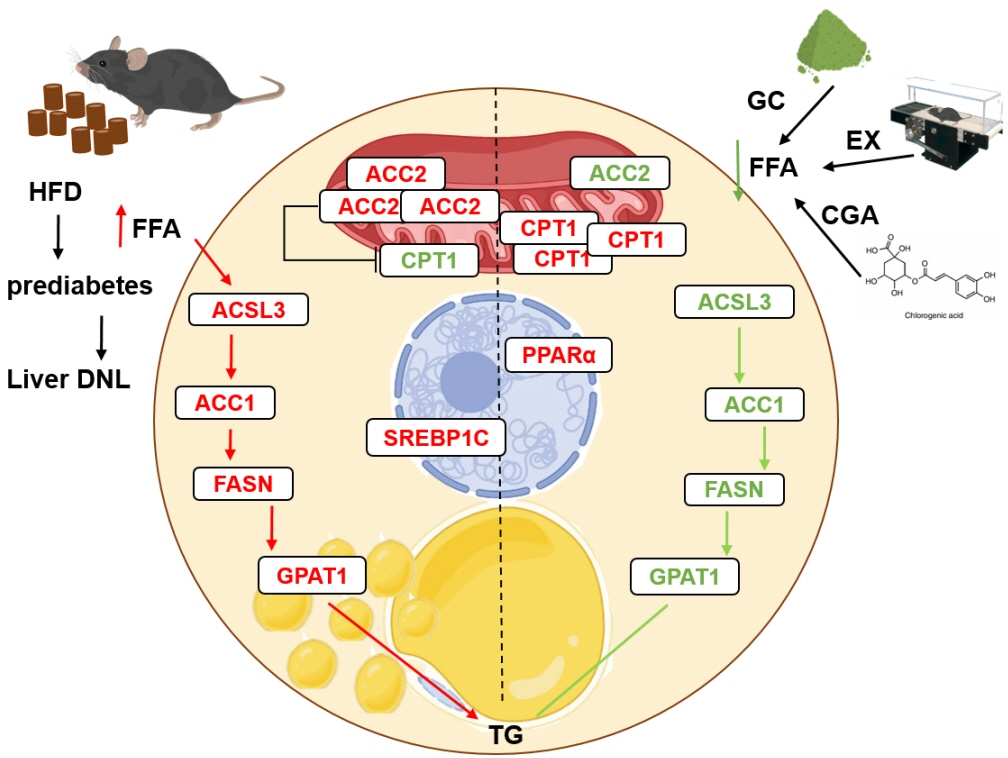
Since prediabetes is a risk factor for metabolic syndromes, it is important to promote a healthy lifestyle to prevent prediabetes. This study aimed to determine the effects of green coffee (GC), chlorogenic acid (CGA) intake, and exercise training (EX) on hepatic lipid metabolism in prediabetes male C57BL/6 mice.
Methods
Forty-nine mice were randomly divided into two groups feeding with a normal diet (n=7) or a high-fat diet (HFD, n=42) for 12 weeks. Then, HFD mice were further divided into six groups (n=7/group): control (pre-D), GC, CGA, EX, GC+EX, and CGA+EX. After additional 10 weeks under the same diet, plasma, and liver samples were obtained.
Results
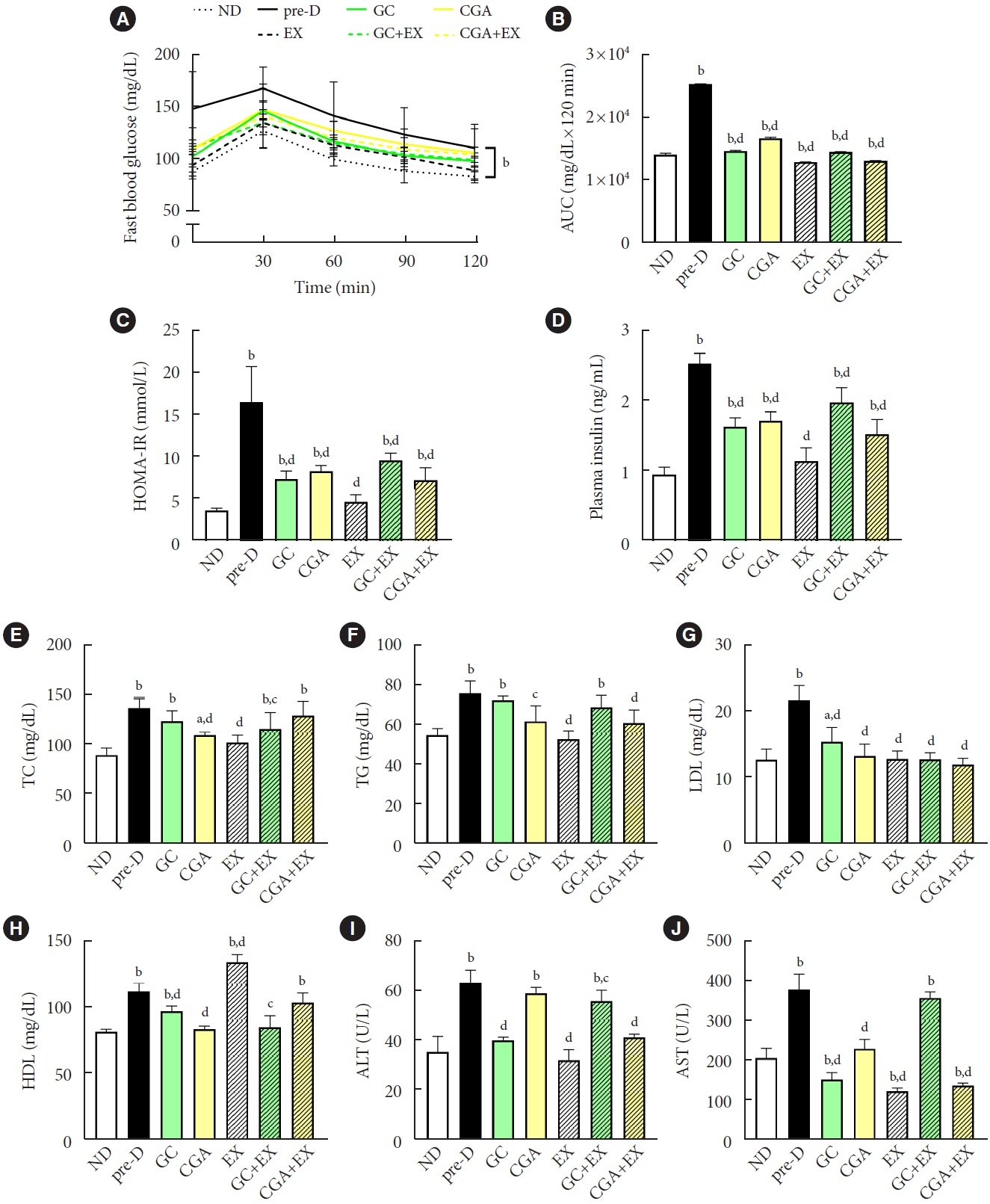
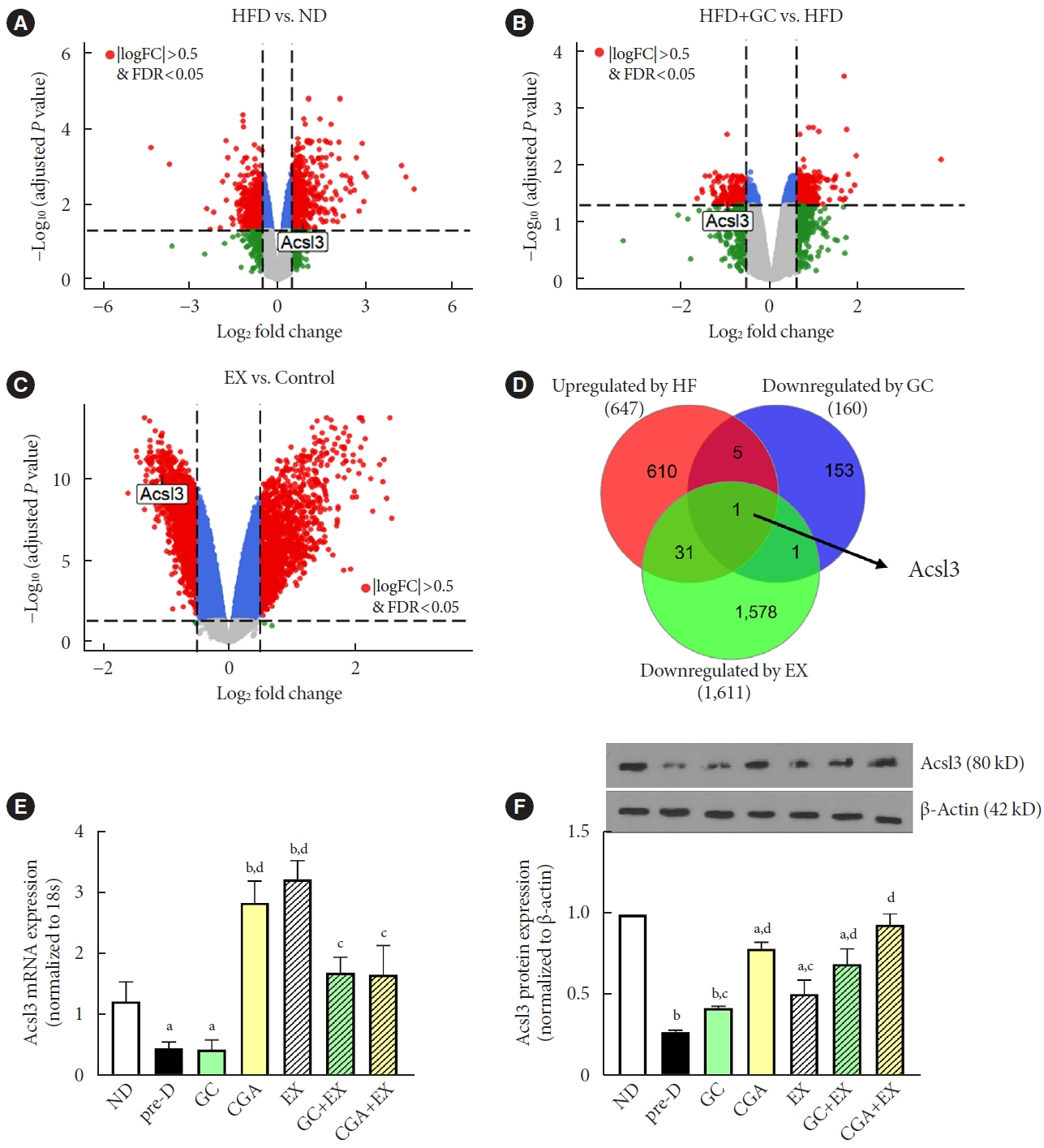
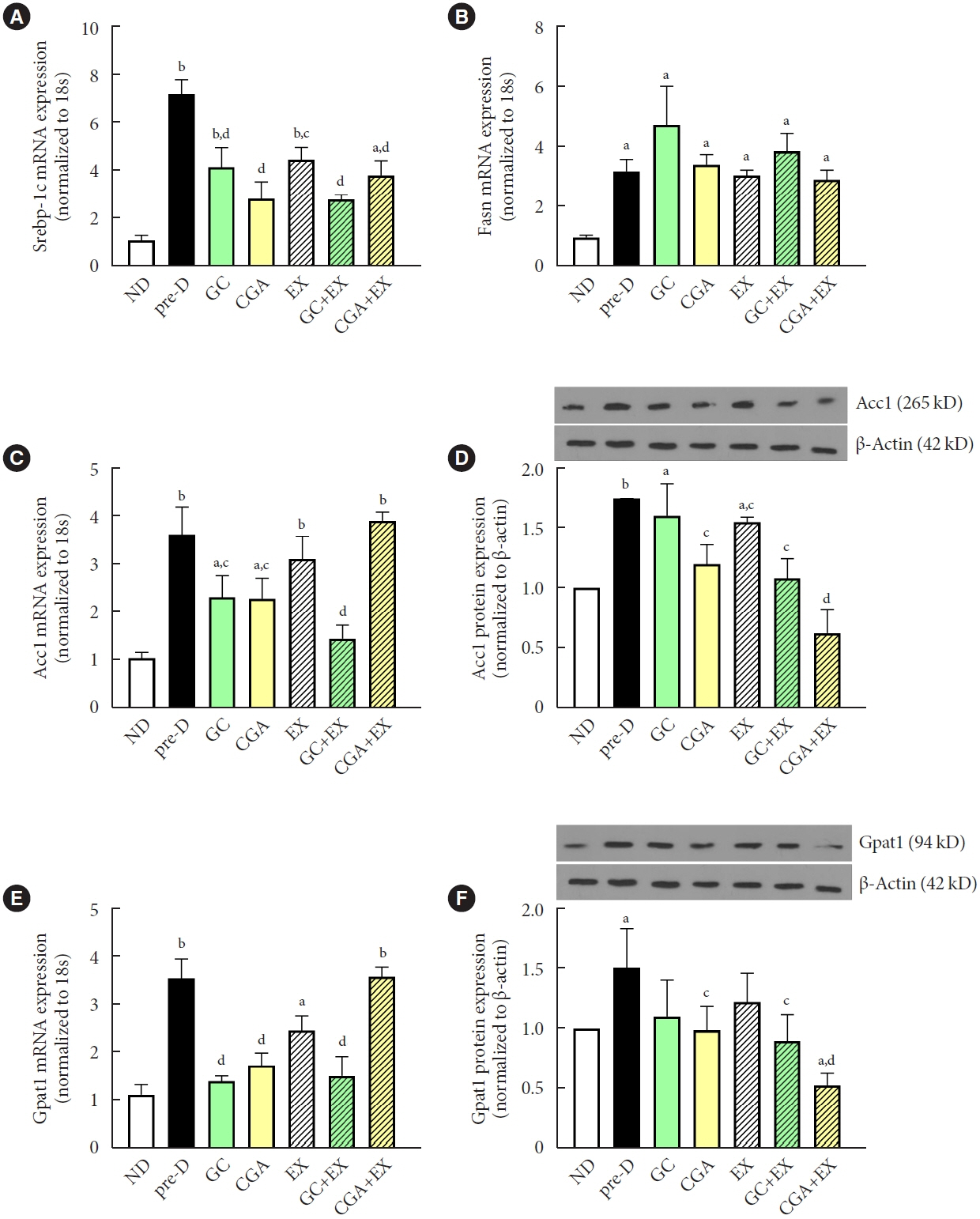
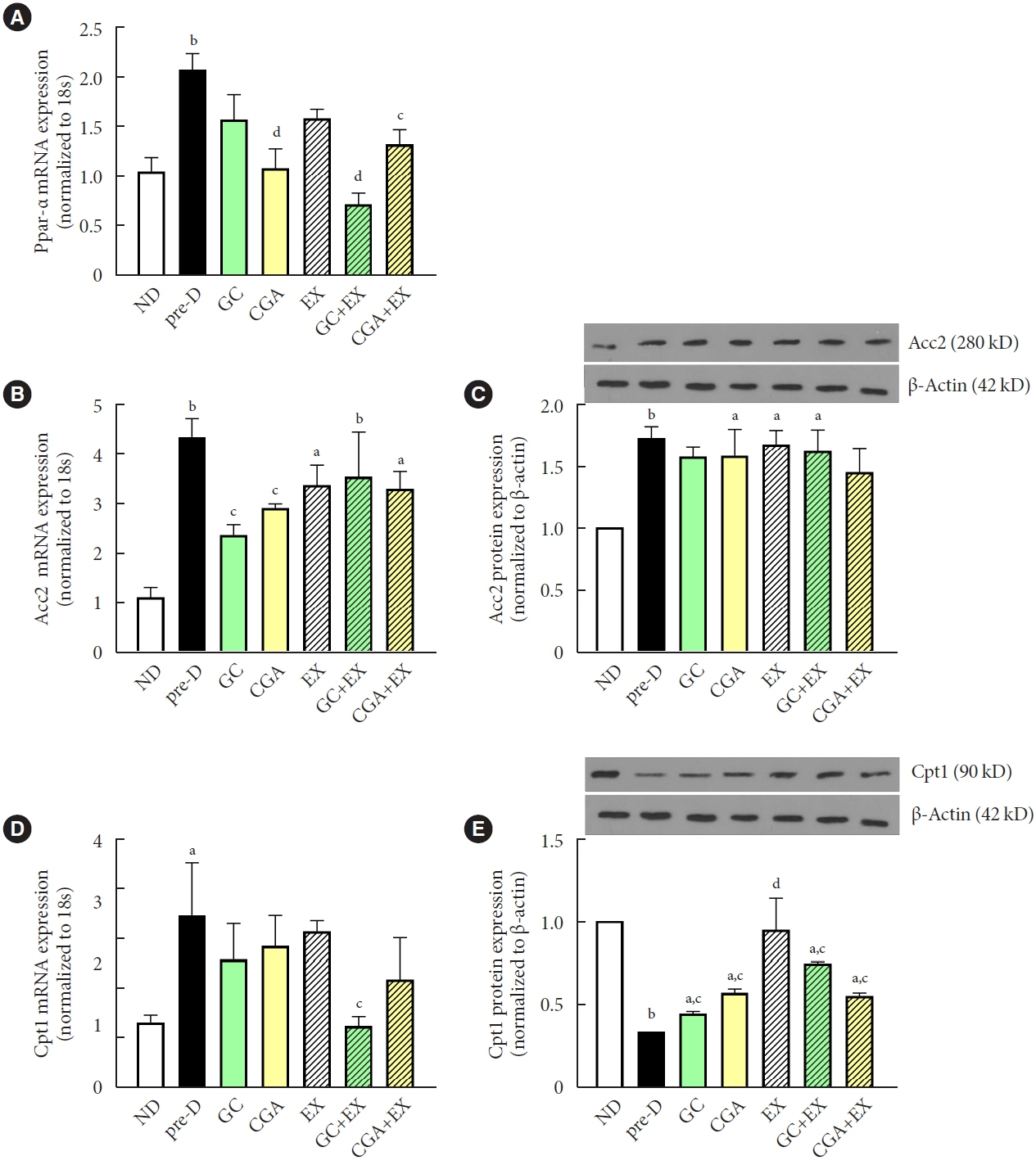
HFD-induced prediabetes conditions with increases in body weight, glucose, insulin, insulin resistance, and lipid profiles were alleviated in all treatment groups. Acsl3, a candidate gene identified through an in silico approach, was lowered in the pre-D group, while treatments partly restored it. HFD induced adverse alterations of de novo lipogenesis- and β oxidation-associated molecules in the liver. However, GC and CGA supplementation and EX reversed or ameliorated these changes. In most cases, GC or CGA supplementation combined with EX has no synergistic effect and the GC group had similar results to the CGA group.
Conclusion
These findings suggest that regular exercise is an effective non-therapeutic approach for prediabetes, and CGA supplementation could be an alternative to partially mimic the beneficial effects of exercise on prediabetes.
Keyword
Figure
Reference
-
1. Loria P, Lonardo A, Anania F. Liver and diabetes: a vicious circle. Hepatol Res. 2013; 43:51–64.
Article2. Zhang CH, Zhou BG, Sheng JQ, Chen Y, Cao YQ, Chen C. Molecular mechanisms of hepatic insulin resistance in nonalcoholic fatty liver disease and potential treatment strategies. Pharmacol Res. 2020; 159:104984.
Article3. Li YP, Xiao J, Liang X, Pei Y, Han XF, Li CX, et al. DPP-4 inhibition resembles exercise in preventing type 2 diabetes development by inhibiting hepatic protein kinase Cε expression in a mouse model of hyperinsulinemia. J Int Med Res. 2020; 48:300060520934635.4. Zand A, Ibrahim K, Patham B. prediabetes: why should we care? Methodist Debakey Cardiovasc J. 2018; 14:289–97.
Article5. Huang L, Fang Y, Tang L. Comparisons of different exercise interventions on glycemic control and insulin resistance in preDiabetes: a network meta-analysis. BMC Endocr Disord. 2021; 21:181.
Article6. Lambert K, Hokayem M, Thomas C, Fabre O, Cassan C, Bourret A, et al. Combination of nutritional polyphenols supplementation with exercise training counteracts insulin resistance and improves endurance in high-fat diet-induced obese rats. Sci Rep. 2018; 8:2885.
Article7. Warner SO, Yao MV, Cason RL, Winnick JJ. Exercise-induced improvements to whole body glucose metabolism in type 2 diabetes: the essential role of the liver. Front Endocrinol (Lausanne). 2020; 11:567.
Article8. Affonso RC, Voytena AP, Fanan S, Pitz H, Coelho DS, Horstmann AL, et al. Phytochemical composition, antioxidant activity, and the effect of the aqueous extract of coffee (Coffea arabica L.) bean residual press cake on the skin wound healing. Oxid Med Cell Longev. 2016; 2016:1923754.9. Abbass MMS, El-Baz DA. The effect of daily intake of green coffee bean extract as compared to Agiolax on the alveolar bone of albino rats. Dent Med Probl. 2018; 55:125–31.
Article10. Ricci A, Parpinello GP, Versari A. The nutraceutical impact of polyphenolic composition in commonly consumed green tea, green coffee and red wine beverages: a review. Recent Adv Food Sci Nutr Res. 2018; 1:12–27.11. Lecoultre V, Carrel G, Egli L, Binnert C, Boss A, MacMillan EL, et al. Coffee consumption attenuates short-term fructose-induced liver insulin resistance in healthy men. Am J Clin Nutr. 2014; 99:268–75.
Article12. Liu M, Qin J, Cong J, Yang Y. Chlorogenic acids inhibit adipogenesis: implications of Wnt/β-catenin signaling pathway. Int J Endocrinol. 2021; 2021:2215274.
Article13. Kong L, Xu M, Qiu Y, Liao M, Zhang Q, Yang L, et al. Chlorogenic acid and caffeine combination attenuates adipogenesis by regulating fat metabolism and inhibiting adipocyte differentiation in 3T3-L1 cells. J Food Biochem. 2021; 45:e13795.
Article14. Sanchez MB, Miranda-Perez E, Verjan JC, de Los Angeles Fortis Barrera M, Perez-Ramos J, Alarcon-Aguilar FJ. Potential of the chlorogenic acid as multitarget agent: insulin-secretagogue and PPAR α/γ dual agonist. Biomed Pharmacother. 2017; 94:169–75.
Article15. Hao S, Xiao Y, Lin Y, Mo Z, Chen Y, Peng X, et al. Chlorogenic acid-enriched extract from Eucommia ulmoides leaves inhibits hepatic lipid accumulation through regulation of cholesterol metabolism in HepG2 cells. Pharm Biol. 2016; 54:251–9.16. Santana-Galvez J, Cisneros-Zevallos L, Jacobo-Velazquez DA. Chlorogenic acid: recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules. 2017; 22:358.
Article17. Naveed M, Hejazi V, Abbas M, Kamboh AA, Khan GJ, Shumzaid M, et al. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed Pharmacother. 2018; 97:67–74.
Article18. Abdollahi M, Marandi SM, Ghaedi K, Safaeinejad Z, Kazeminasab F, Shirkhani S, et al. Insulin-related liver pathways and the therapeutic effects of aerobic training, green coffee, and chlorogenic acid supplementation in pre-Diabetic mice. Oxid Med Cell Longev. 2022; 2022:5318245.
Article19. Seo DY, Park SH, Marquez J, Kwak HB, Kim TN, Bae JH, et al. Hepatokines as a molecular transducer of exercise. J Clin Med. 2021; 10:385.
Article20. Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol. 2017; 8:1–8.21. Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, et al. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2007; 20:351–8.
Article22. Kojta I, Zabielski P, Roszczyc-Owsiejczuk K, Imierska M, Sokolowska E, Blachnio-Zabielska A. GPAT gene silencing in muscle reduces diacylglycerols content and improves insulin action in diet-induced insulin resistance. Int J Mol Sci. 2020; 21:7369.
Article23. Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res. 2009; 50(Suppl):S74–9.
Article24. Alves CC, Waitzberg DL, de Andrade LS, Dos Santos Aguiar L, Reis MB, Guanabara CC, et al. Prebiotic and synbiotic modifications of beta oxidation and lipogenic gene expression after experimental hypercholesterolemia in rat liver. Front Microbiol. 2017; 8:2010.
Article25. Stevanovic J, Beleza J, Coxito P, Ascensao A, Magalhaes J. Physical exercise and liver “fitness”: role of mitochondrial function and epigenetics-related mechanisms in non-alcoholic fatty liver disease. Mol Metab. 2020; 32:1–14.
Article26. Meng S, Cao J, Feng Q, Peng J, Hu Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: a review. Evid Based Complement Alternat Med. 2013; 2013:801457.
Article27. Wang Y, Xu D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017; 16:132.
Article28. Ahmadvand H, Davoodi T, Babaeenezhad E. Effects of caffeic acid on serum lipid profile and atherogenic index in alloxaninduced diabetic rats. Herb Med J. 2017; 2:3–8.29. Hayek T, Ito Y, Azrolan N, Verdery RB, Aalto-Setala K, Walsh A, et al. Dietary fat increases high density lipoprotein (HDL) levels both by increasing the transport rates and decreasing the fractional catabolic rates of HDL cholesterol ester and apolipoprotein (Apo) A-I: presentation of a new animal model and mechanistic studies in human Apo A-I transgenic and control mice. J Clin Invest. 1993; 91:1665–71.
Article30. Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014; 44:211–21.
Article31. Ilmiawati C, Fitri F, Rofinda ZD, Reza M. Green coffee extract modifies body weight, serum lipids and TNF-α in high-fat diet-induced obese rats. BMC Res Notes. 2020; 13:208.
Article32. Dungubat E, Watabe S, Togashi-Kumagai A, Watanabe M, Kobayashi Y, Harada N, et al. Effects of caffeine and chlorogenic acid on nonalcoholic steatohepatitis in mice induced by choline-deficient, L-amino acid-defined, high-fat diet. Nutrients. 2020; 12:3886.
Article33. la Fuente FP, Quezada L, Sepulveda C, Monsalves-Alvarez M, Rodriguez JM, Sacristan C, et al. Exercise regulates lipid droplet dynamics in normal and fatty liver. Biochim Biophys Acta Mol Cell Biol Lipids. 2019; 1864:158519.
Article34. Cui B, Liu S, Lin X, Wang J, Li S, Wang Q, et al. Effects of Lycium barbarum aqueous and ethanol extracts on high-fat-diet induced oxidative stress in rat liver tissue. Molecules. 2011; 16:9116–28.
Article35. Zhou Y, Li H, Xia N. The interplay between adipose tissue and vasculature: role of oxidative stress in obesity. Front Cardiovasc Med. 2021; 8:650214.
Article36. Padanad MS, Konstantinidou G, Venkateswaran N, Melegari M, Rindhe S, Mitsche M, et al. Fatty acid oxidation mediated by Acyl-CoA synthetase long chain 3 is required for mutant KRAS lung tumorigenesis. Cell Rep. 2016; 16:1614–28.
Article37. Etxeberria U, Castilla-Madrigal R, Lostao MP, Martinez JA, Milagro FI. Trans-resveratrol induces a potential anti-lipogenic effect in lipopolysaccharide-stimulated enterocytes. Cell Mol Biol (Noisy-le-grand). 2015; 61:9–16.38. Smith GI, Shankaran M, Yoshino M, Schweitzer GG, Chondronikola M, Beals JW, et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 2020; 130:1453–60.
Article39. Muscella A, Stefano E, Lunetti P, Capobianco L, Marsigliante S. The regulation of fat metabolism during aerobic exercise. Biomolecules. 2020; 10:1699.
Article40. Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015; 62:720–33.
Article41. Gavito AL, Bautista D, Suarez J, Badran S, Arco R, Pavon FJ, et al. Chronic IL-6 administration desensitizes IL-6 response in liver, causes hyperleptinemia and aggravates steatosis in dietinduced-obese mice. PLoS One. 2016; 11:e0157956.
Article42. Rui L. Energy metabolism in the liver. Compr Physiol. 2014; 4:177–97.
Article43. Wang Y, Yu W, Li S, Guo D, He J, Wang Y. Acetyl-CoA carboxylases and diseases. Front Oncol. 2022; 12:836058.
Article44. Murase T, Misawa K, Minegishi Y, Aoki M, Ominami H, Suzuki Y, et al. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. Am J Physiol Endocrinol Metab. 2011; 300:E122–33.
Article45. Guimaraes JC, Rocha M, Arkin AP. Transcript level and sequence determinants of protein abundance and noise in Escherichia coli. Nucleic Acids Res. 2014; 42:4791–9.
Article46. Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003; 4:117.47. Yuan T, Wu D, Sun K, Tan X, Wang J, Zhao T, et al. Anti-fatigue activity of aqueous extracts of Sonchus arvensis L. in exercise trained mice. Molecules. 2019; 24:1168.
Article48. Panza VP, Diefenthaeler F, Tamborindeguy AC, Camargo Cde Q, de Moura BM, Brunetta HS, et al. Effects of mate tea consumption on muscle strength and oxidative stress markers after eccentric exercise. Br J Nutr. 2016; 115:1370–8.
Article49. Ommati MM, Farshad O, Mousavi K, Khalili M, Jamshidzadeh A, Heidari R. Chlorogenic acid supplementation improves skeletal muscle mitochondrial function in a rat model of resistance training. Biologia. 2020; 75:1221–30.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chlorogenic Acid Supplementation Improves Multifocal Electroretinography in Patients with Retinitis Pigmentosa
- Folic acid supplementation prevents high fructose-induced non-alcoholic fatty liver disease by activating the AMPK and LKB1 signaling pathways
- Identification of proteins regulated by chlorogenic acid in an ischemic animal model: a proteomic approach
- Effects of quercetin on the improvement of lipid metabolism through regulating hepatic AMPK and microRNA-21 in high cholesterol diet-fed mice
- The Spermatogenic Effect of Yacon Extract and Its Constituents and Their Inhibition Effect of Testosterone Metabolism