Restor Dent Endod.
2021 Nov;46(4):e47. 10.5395/rde.2021.46.e47.
Laboratory model to evaluate efficacy of an experimental titanium oxide nanofibers bleaching agent
- Affiliations
-
- 1Division of General Dentistry, Loma Linda University School of Dentistry, Loma Linda, California, USA
- 2Department of Biochemistry, Loma Linda University School of Medicine, Loma Linda, California, USA
- KMID: 2548091
- DOI: http://doi.org/10.5395/rde.2021.46.e47
Abstract
Objectives
This study aimed to use a laboratory model to evaluate the efficacy of an experimental bleaching agent.
Materials and Methods
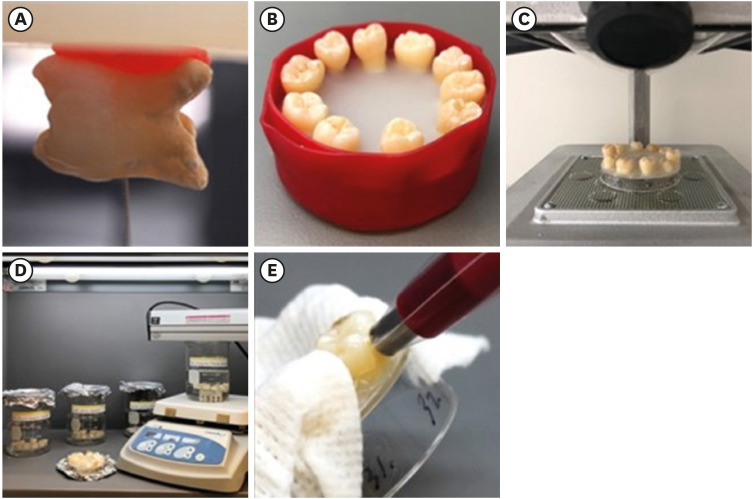
The model used human extracted molars that were treated and measured for bleaching efficacy. Teeth (n = 50) were distributed into 5 groups: Negative control (NC): immersion in water for 8 hours; Nanofibers (NFs): Experimental titanium dioxide nanofibers with stirring and light activation for 8 hours; Whitestrips (WS): Crest 3D White Glamorous White Whitestrips, 2 applications daily for 30 minutes, 14 days; 1% hydrogen peroxide (HP) standard: 1% hydrogen peroxide for 8 hours; and 30% HP standard: 30% hydrogen peroxide for 8 hours. Instrumental measurements were performed using a spectrophotometer. Results were recorded at baseline, 1-day post-bleaching, and 1-week post-bleaching. Kruskal-Wallis procedure was used to determine differences in color change. Pearson correlation was used to evaluate the relationship between visual and instrumental measurements. Tests of hypotheses were 2-sided with alpha = 0.05.
Results
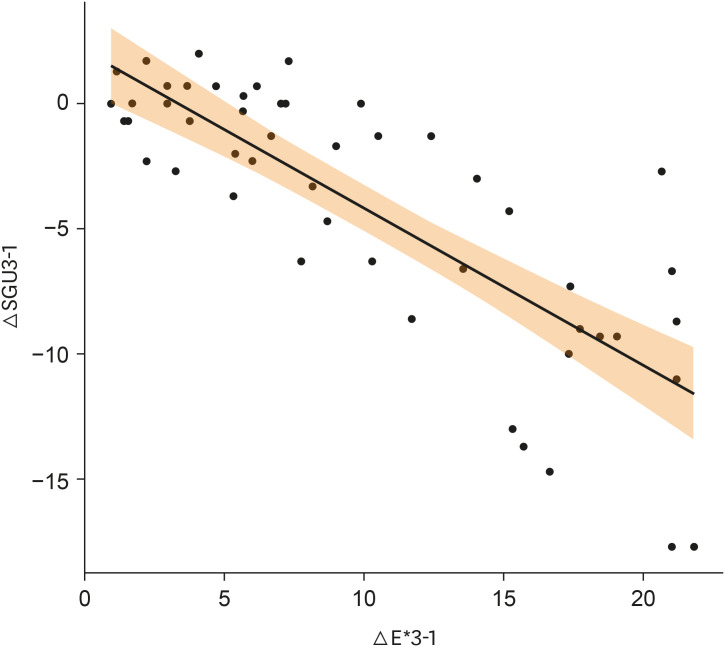
There was no significant difference in color parameters (L1, a1, b1, and shade guide units [SGU]) at baseline (p > 0.05). There was a significant difference among the groups for overall color change (ΔE*ab) and change in shade guide units (ΔSGU) at 1-day and 1-week post-bleaching (p < 0.05). The higher the HP concentration, the higher the color change as expressed in ΔSGU and ΔE*ab. The negative control exceeded the perceptibility threshold of ΔE* = 1.2 regardless of time point. NFs showed a decrease in chroma, but were not statistically different compared to the negative control.
Conclusions
The laboratory model was successful in screening an experimental bleaching agent.
Keyword
Figure
Reference
-
1. Lazarchik DA, Haywood VB. Use of tray-applied 10 percent carbamide peroxide gels for improving oral health in patients with special-care needs. J Am Dent Assoc. 2010; 141:639–646. PMID: 20516093.
Article2. Kwon SR, Wertz PW. Review of the mechanism of tooth whitening. J Esthet Restor Dent. 2015; 27:240–257. PMID: 25969131.
Article3. Vaz MM, Lopes LG, Cardoso PC, Souza JB, Batista AC, Costa NL, Torres ÉM, Estrela C. Inflammatory response of human dental pulp to at-home and in-office tooth bleaching. J Appl Oral Sci. 2016; 24:509–517. PMID: 27812622.
Article4. Silva-Costa RSGD, Ribeiro AEL, Assunção IV, Araújo Júnior RF, Araújo AA, Guerra GCB, Borges BCD. In-office tooth bleaching with 38% hydrogen peroxide promotes moderate/severe pulp inflammation and production of ll-1β, TNF-β, GPX, FGF-2 and osteocalcin in rats. J Appl Oral Sci. 2018; 26:e20170367. PMID: 29898175.5. Munro IC, Williams GM, Heymann HO, Kroes R. Tooth whitening products and the risk of oral cancer. Food Chem Toxicol. 2006; 44:301–315. PMID: 16198468.
Article6. International Standard Organization. ISO Standards [Internet]. Geneva: International Standard Organization;c2021. cited 2018 Nov 30. Available from: https://www.iso.org/standards.html.7. International Organization for Standardization. ISO 28399 Dentistry – External tooth bleaching products. Geneva: International Standard Organization;2021.8. Paravina RD, Pérez MM, Ghinea R. Acceptability and perceptibility thresholds in dentistry: a comprehensive review of clinical and research applications. J Esthet Restor Dent. 2019; 31:103–112. PMID: 30891913.
Article9. International Organization for Standardization. ISO/TR 28642 Dentistry – Guidance on colour measurement. Geneva: International Standard Organization;2016.10. Kwon SR, Cortez E, Wang M, Jagwani M, Oyoyo U, Li Y. Systematic review of in vitro studies evaluating tooth bleaching efficacy. Am J Dent. 2020; 33:17–24. PMID: 32056410.11. European Union. Council Directive 2011/84/EU of 20 September 2011 amending Directive 76/768/EEC, concerning cosmetic products, for the purpose of adapting Annex III thereto to technical progress. Off J Eur Union. 2019; 54:36–38.12. Greenwall-Cohen J, Francois P, Silikas N, Greenwall L, Le Goff S, Attal JP. The safety and efficacy of ‘over the counter’ bleaching products in the UK. Br Dent J. 2019; 226:271–276. PMID: 30787399.
Article13. Al-Omiri MK, Lamfon HA, Al Nazeh AA, Kielbassa AM, Lynch E. Randomized clinical trial on the comparison of bleaching outcomes using either ozone or hydrogen peroxide. Quintessence Int. 2018; 49:625–634. PMID: 30027174.14. Santana MS, Bridi EC, Navarro RS, de Lima CJ, Fernandes AB, do Amaral FL, França FM, Turssi CP, Basting RT. Dental bleaching with ozone: effects on color and enamel microhardness. Acta Odontol Latinoam. 2016; 29:68–75. PMID: 27701501.15. Aykut-Yetkiner A, Ertuğrul F, Eden E, Aladağ A, Ergin E, Özcan M. Color assessment after bleaching with hydrogen peroxide versus ozone: a randomized controlled clinical trial. Gen Dent. 2017; 65:e12–e17.16. Saita M, Kobayashi K, Yoshino F, Hase H, Nonami T, Kimoto K, Lee MC. ESR investigation of ROS generated by H2O2 bleaching with TiO2 coated HAp. Dent Mater J. 2012; 31:458–464. PMID: 22673461.
Article17. Chi C, Springer BN, Walemba E, Nick KE, Perry CC, Kwon SR. Titanium-oxide nanoparticles and nanofibers used alone or with UV light activation. CDA J. 2019; 47:777–782.
Article18. Shellis RP. Effects of a supersaturated pulpal fluid on the formation of caries-like lesions on the roots of human teeth. Caries Res. 1994; 28:14–20. PMID: 8124692.
Article19. Ontiveros JC, Paravina RD. Color change of vital teeth exposed to bleaching performed with and without supplementary light. J Dent. 2009; 37:840–847. PMID: 19625119.
Article20. Commission Internationale de l'Eclairage. Colorimetry-technical report. Vienna: Bureau Central de la CIE;1986.21. Pascal C, Mehta P, Pascal J, Oyoyo U, Kwon S. A laboratory model to evaluate bleaching efficacy in stained vs non-stained human teeth. In : ADA Standards Committee on Dental Products and the U.S. Technical Advisory Group to the International Organization for Standardization Technical Committee 106 on Dentistry meeting; 2019 Jun 19; Vancouver, Canada.22. ADA Seal of Acceptance. Bleaching products [Internet]. Chicago, IL: American Dental Association;c2021. cited 2020 Sep. Available from: https://www.ada.org/en/science-research/ada-seal-of-acceptance/ada-seal-products/product-category?category=Bleaching+Products.23. Kinser PA, Robins JL. Control group design: enhancing rigor in research of mind-body therapies for depression. Evid Based Complement Alternat Med. 2013; 2013:140467. PMID: 23662111.
Article24. Horwitz W. Good laboratory practices in analytical chemistry. Anal Chem. 1978; 50:521–524. PMID: 637302.
Article25. Kwon SR, Meharry M, Oyoyo U, Li Y. Efficacy of do-it-yourself whitening as compared to conventional tooth whitening modalities: an in vitro study. Oper Dent. 2015; 40:E21–E27. PMID: 25279797.26. Allard MM, Merlos SN, Springer BN, Cooper J, Zhang GY, Boskovic DS, Kwon SR, Nick KE, Perry CC. Role of TiO2 anatase surface morphology on organophosphorus interfacial chemistry. J Phys Chem C. 2018; 122:29237–29248.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The evaluation of clinical efficacy and longevity of home bleaching without combined application of In-office bleaching
- Effects of Citrus limon Extract on Oxidative Stress-Induced Nitric Oxide Generation and Bovine Teeth Bleaching
- Evaluation of at-home bleaching protocol with application on different surfaces: bleaching efficacy and hydrogen peroxide permeability
- Effect of vital tooth bleaching agent on dentin bonding
- Diagnosis and treatment of sodium hypochlorite poisoning with ingestion of household bleaching agents