Int J Stem Cells.
2023 Nov;16(4):406-414. 10.15283/ijsc23027.
Low-Intensity Pulsed Ultrasound Promotes BMP9 Induced Osteoblastic Differentiation in Rat Dedifferentiated Fat Cells
- Affiliations
-
- 1Department of Periodontology, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan
- 2Division of Preventive Dentistry, Department of Community Social Dentistry, Graduate School of Dentistry, Tohoku University, Miyagi, Japan
- KMID: 2548004
- DOI: http://doi.org/10.15283/ijsc23027
Abstract
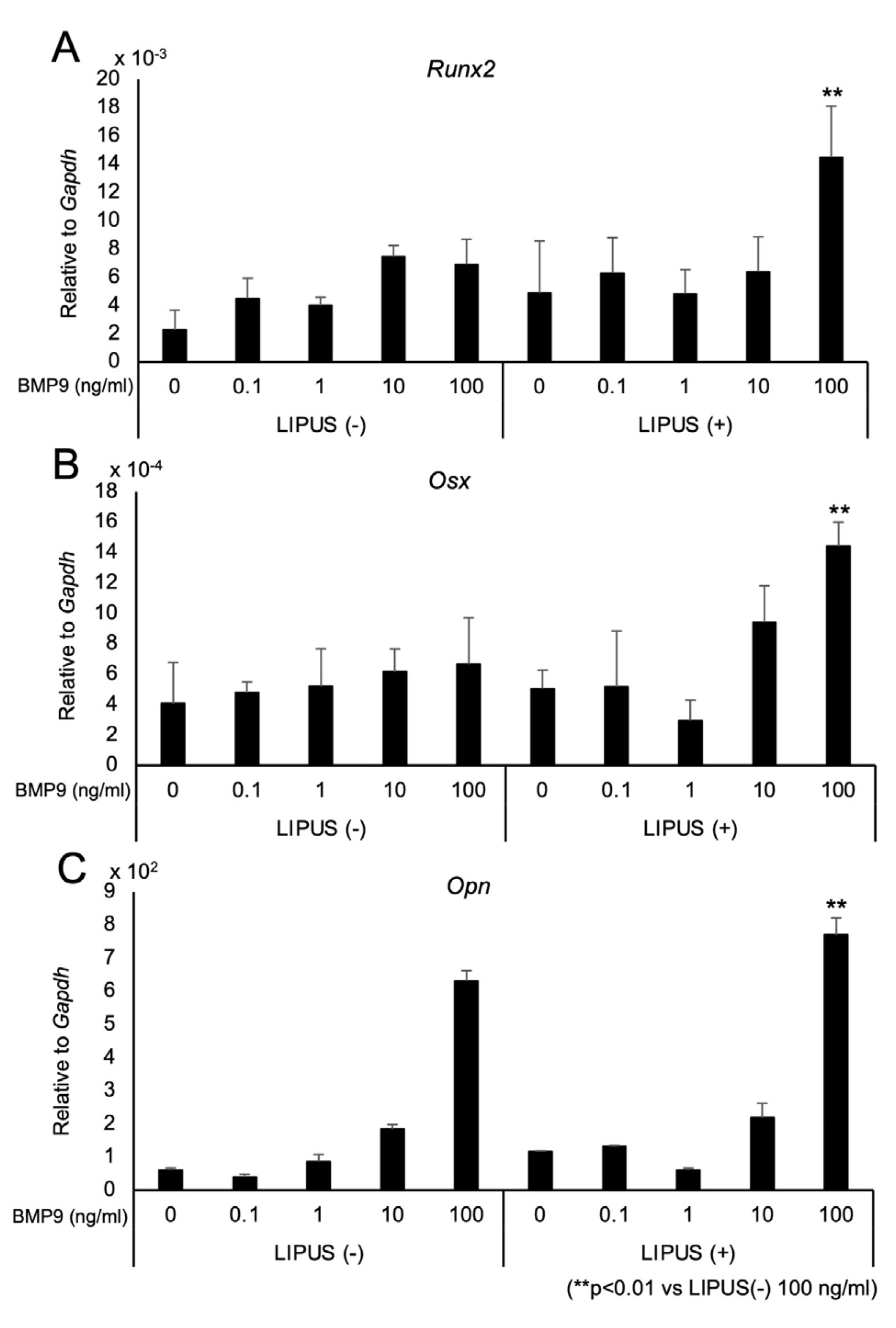
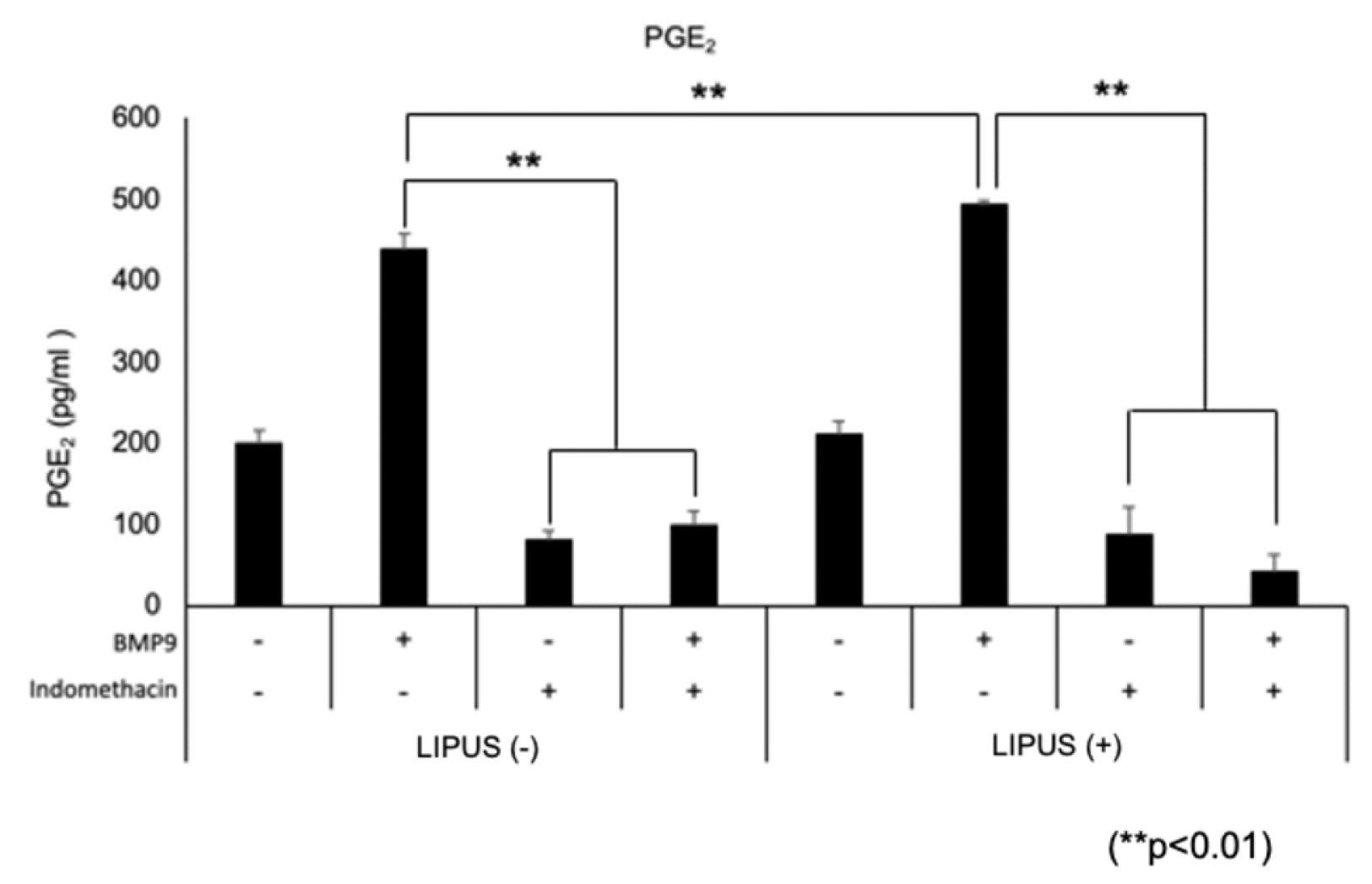
- Dedifferentiated fat cells (DFATs) isolated from mature adipocytes have a multilineage differentiation capacity similar to mesenchymal stem cells and are considered as promising source of cells for tissue engineering. Bone morphogenetic protein 9 (BMP9) and low-intensity pulsed ultrasound (LIPUS) have been reported to stimulate bone formation both in vitro and in vivo. However, the combined effect of BMP9 and LIPUS on osteoblastic differentiation of DFATs has not been studied. After preparing DFATs from mature adipose tissue from rats, DFATs were treated with different doses of BMP9 and/or LIPUS. The effects on osteoblastic differentiation were assessed by changes in alkaline phosphatase (ALP) activity, mineralization/calcium deposition, and expression of bone related genes; Runx2, osterix, osteopontin. No significant differences for ALP activity, mineralization deposition, as well as expression for bone related genes were observed by LIPUS treatment alone while treatment with BMP9 induced osteoblastic differentiation of DFATs in a dose dependent manner. Further, co-treatment with BMP9 and LIPUS significantly increased osteoblastic differentiation of DFATs compared to those treated with BMP9 alone. In addition, upregulation for BMP9-receptor genes was observed by LIPUS treatment. Indomethacin, an inhibitor of prostaglandin synthesis, significantly inhibited the synergistic effect of BMP9 and LIPUS co-stimulation on osteoblastic differentiation of DFATs. LIPUS promotes BMP9 induced osteoblastic differentiation of DFATs in vitro and prostaglandins may be involved in this mechanism.
Keyword
Figure
Reference
-
References
1. Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, Ryu J, Mugishima H. 2008; Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 215:210–222. DOI: 10.1002/jcp.21304. PMID: 18064604.2. Saler M, Caliogna L, Botta L, Benazzo F, Riva F, Gastaldi G. 2017; hASC and DFAT, multipotent stem cells for regenera-tive medicine: a comparison of their potential differentia-tion in vitro. Int J Mol Sci. 18:2699. DOI: 10.3390/ijms18122699. PMID: 29236047. PMCID: PMC5751300. PMID: c32655091c2a4feaa3e584e5937064cd.
Article3. Sakamoto F, Hashimoto Y, Kishimoto N, Honda Y, Matsu-moto N. 2015; The utility of human dedifferentiated fat cells in bone tissue engineering in vitro. Cytotechnology. 67:75–84. DOI: 10.1007/s10616-013-9659-y. PMID: 24306271. PMCID: PMC4294844.
Article4. Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. 2011; Bone morphogenetic proteins: a critical review. Cell Signal. 23:609–620. DOI: 10.1016/j.cellsig.2010.10.003. PMID: 20959140.
Article5. Cheng SL, Lou J, Wright NM, Lai CF, Avioli LV, Riew KD. 2001; In Vitro andIn Vivo induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene. Calcif Tissue Int. 68:87–94. DOI: 10.1007/BF02678146. PMID: 27696150.
Article6. Krebsbach PH, Gu K, Franceschi RT, Rutherford RB. 2000; Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 11:1201–1210. DOI: 10.1089/10430340050015248. PMID: 10834621.
Article7. Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, Vanichakarn P, Park JY, Li Y, Haydon RC, He TC. 2004; Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 11:1312–1320. DOI: 10.1038/sj.gt.3302298. PMID: 15269709.
Article8. Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatko-wski JP, Park JY, He TC. 2003; Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am. 85:1544–1552. DOI: 10.2106/00004623-200308000-00017. PMID: 12925636.
Article9. Wang Y, Hong S, Li M, Zhang J, Bi Y, He Y, Liu X, Nan G, Su Y, Zhu G, Li R, Zhang W, Wang J, Zhang H, Kong Y, Shui W, Wu N, He Y, Chen X, Luu HH, Haydon RC, Shi LL, He TC, Qin J. 2013; Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J Orthop Res. 31:1796–1803. DOI: 10.1002/jor.22427. PMID: 23861103.
Article10. Doan N, Reher P, Meghji S, Harris M. 1999; In vitro effects of therapeutic ultrasound on cell proliferation, protein synthesis, and cytokine production by human fibroblasts, osteoblasts, and monocytes. J Oral Maxillofac Surg. 57:409–419. discussion 420DOI: 10.1016/S0278-2391(99)90281-1. PMID: 10199493.
Article11. Xie S, Jiang X, Wang R, Xie S, Hua Y, Zhou S, Yang Y, Zhang J. 2019; Low-intensity pulsed ultrasound promotes the proliferation of human bone mesenchymal stem cells by activating PI3K/AKt signaling pathways. J Cell Biochem. 120:15823–15833. DOI: 10.1002/jcb.28853. PMID: 31090943.
Article12. Li H, Zhou J, Zhu M, Ying S, Li L, Chen D, Li J, Song J. 2021; Low-intensity pulsed ultrasound promotes the formation of periodontal ligament stem cell sheets and ectopic periodontal tissue regeneration. J Biomed Mater Res A. 109:1101–1112. DOI: 10.1002/jbm.a.37102. PMID: 32964617.
Article13. Busse JW, Bhandari M, Kulkarni AV, Tunks E. 2002; The effect of low-intensity pulsed ultrasound therapy on time to fracture healing: a meta-analysis. CMAJ. 166:437–441.14. Kokubu T, Matsui N, Fujioka H, Tsunoda M, Mizuno K. 1999; Low intensity pulsed ultrasound exposure increases prostaglandin E2 production via the induction of cyclooxygenase-2 mRNA in mouse osteoblasts. Biochem Biophys Res Commun. 256:284–287. DOI: 10.1006/bbrc.1999.0318. PMID: 10079177.
Article15. Tang CH, Yang RS, Huang TH, Lu DY, Chuang WJ, Huang TF, Fu WM. 2006; Ultrasound stimulates cyclooxygenase-2 expression and increases bone formation through integrin, focal adhesion kinase, phosphatidylinositol 3-kinase, and Akt pathway in osteoblasts. Mol Pharmacol. 69:2047–2057. DOI: 10.1124/mol.105.022160. PMID: 16540596.
Article16. Nakamura T, Shinohara Y, Momozaki S, Yoshimoto T, Noguchi K. 2013; Co-stimulation with bone morphogenetic protein-9 and FK506 induces remarkable osteoblastic differentiation in rat dedifferentiated fat cells. Biochem Biophys Res Commun. 440:289–294. DOI: 10.1016/j.bbrc.2013.09.073. PMID: 24064349.
Article17. Imafuji T, Shirakata Y, Shinohara Y, Nakamura T, Noguchi K. 2021; Enhanced bone formation of calvarial bone defects by low-intensity pulsed ultrasound and recombinant human bone morphogenetic protein-9: a preliminary experimental study in rats. Clin Oral Investig. 25:5917–5927. DOI: 10.1007/s00784-021-03897-6. PMID: 33755786.
Article18. Jumabay M, Matsumoto T, Yokoyama S, Kano K, Kusumi Y, Masuko T, Mitsumata M, Saito S, Hirayama A, Mugishima H, Fukuda N. 2009; Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J Mol Cell Cardiol. 47:565–575. DOI: 10.1016/j.yjmcc.2009.08.004. PMID: 19686758.
Article19. Sena K, Leven RM, Mazhar K, Sumner DR, Virdi AS. 2005; Early gene response to low-intensity pulsed ultrasound in rat osteoblastic cells. Ultrasound Med Biol. 31:703–708. DOI: 10.1016/j.ultrasmedbio.2005.01.013. PMID: 15866420.
Article20. Angle SR, Sena K, Sumner DR, Virdi AS. 2011; Osteogenic differentiation of rat bone marrow stromal cells by various intensities of low-intensity pulsed ultrasound. Ultrasonics. 51:281–288. DOI: 10.1016/j.ultras.2010.09.004. PMID: 20965537.
Article21. Sakoda K, Nakajima Y, Noguchi K. 2012; Enamel matrix derivative induces production of vascular endothelial cell growth factor in human gingival fibroblasts. Eur J Oral Sci. 120:513–519. DOI: 10.1111/j.1600-0722.2012.00999.x. PMID: 23167467.
Article22. Chen D, Xiang M, Gong Y, Xu L, Zhang T, He Y, Zhou M, Xin L, Li J, Song J. 2019; LIPUS promotes FOXO1 accumulation by downregulating miR-182 to enhance osteogenic differentiation in hPDLCs. Biochimie. 165:219–228. DOI: 10.1016/j.biochi.2019.08.005. PMID: 31401188.
Article23. Kusuyama J, Bandow K, Shamoto M, Kakimoto K, Ohnishi T, Matsuguchi T. 2014; Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J Biol Chem. 289:10330–10344. DOI: 10.1074/jbc.M113.546382. PMID: 24550383. PMCID: PMC4036157.
Article24. Naruse K, Miyauchi A, Itoman M, Mikuni-Takagaki Y. 2003; Distinct anabolic response of osteoblast to low-intensity pulsed ultrasound. J Bone Miner Res. 18:360–369. DOI: 10.1359/jbmr.2003.18.2.360. PMID: 12568414.
Article25. Sant'Anna EF, Leven RM, Virdi AS, Sumner DR. 2005; Effect of low intensity pulsed ultrasound and BMP-2 on rat bone marrow stromal cell gene expression. J Orthop Res. 23:646–652. DOI: 10.1016/j.orthres.2004.09.007. PMID: 15885487.26. Lai CH, Chen SC, Chiu LH, Yang CB, Tsai YH, Zuo CS, Chang WH, Lai WF. 2010; Effects of low-intensity pulsed ultrasound, dexamethasone/TGF-beta1 and/or BMP-2 on the transcriptional expression of genes in human mesenchymal stem cells: chondrogenic vs. osteogenic differentiation. Ultra-sound Med Biol. 36:1022–1033. DOI: 10.1016/j.ultrasmedbio.2010.03.014. PMID: 20510190.
Article27. Han JJ, Yang HJ, Hwang SJ. 2022; Enhanced bone regeneration by bone morphogenetic protein-2 after pretreatment with low-intensity pulsed ultrasound in distraction osteogenesis. Tissue Eng Regen Med. 19:871–886. DOI: 10.1007/s13770-022-00457-1. PMID: 35594008. PMCID: PMC9294106.
Article28. Wijdicks CA, Virdi AS, Sena K, Sumner DR, Leven RM. 2009; Ultrasound enhances recombinant human BMP-2 induced ectopic bone formation in a rat model. Ultrasound Med Biol. 35:1629–1637. DOI: 10.1016/j.ultrasmedbio.2009.04.017. PMID: 19632764.
Article29. Angle SR, Sena K, Sumner DR, Virkus WW, Virdi AS. 2014; Combined use of low-intensity pulsed ultrasound and rhBMP-2 to enhance bone formation in a rat model of critical size defect. J Orthop Trauma. 28:605–611. DOI: 10.1097/BOT.0000000000000067. PMID: 24464096. PMCID: PMC4108582.
Article30. Miyazono K, Kamiya Y, Morikawa M. 2010; Bone morphogenetic protein receptors and signal transduction. J Biochem. 147:35–51. DOI: 10.1093/jb/mvp148. PMID: 19762341.
Article31. Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, Löwik CW, ten Dijke P. 2007; BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 120(Pt 6):964–972. DOI: 10.1242/jcs.002949. PMID: 17311849.
Article32. Luo J, Tang M, Huang J, He BC, Gao JL, Chen L, Zuo GW, Zhang W, Luo Q, Shi Q, Zhang BQ, Bi Y, Luo X, Jiang W, Su Y, Shen J, Kim SH, Huang E, Gao Y, Zhou JZ, Yang K, Luu HH, Pan X, Haydon RC, Deng ZL, He TC. 2010; TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J Biol Chem. 285:29588–29598. DOI: 10.1074/jbc.M110.130518. PMID: 20628059. PMCID: PMC2937990.
Article33. Suzuki A, Takayama T, Suzuki N, Kojima T, Ota N, Asano S, Ito K. 2009; Daily low-intensity pulsed ultrasound stimulates production of bone morphogenetic protein in ROS 17/2.8 cells. J Oral Sci. 51:29–36. DOI: 10.2334/josnusd.51.29. PMID: 19325197.
Article34. Jee WS, Ma YF. 1997; The in vivo anabolic actions of prostaglandins in bone. Bone. 21:297–304. DOI: 10.1016/S8756-3282(97)00147-6. PMID: 9315332.
Article35. Harrison A, Lin S, Pounder N, Mikuni-Takagaki Y. 2016; Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair. Ultrasonics. 70:45–52. DOI: 10.1016/j.ultras.2016.03.016. PMID: 27130989.
Article36. Saini V, Yadav S, McCormick S. 2011; Low-intensity pulsed ultrasound modulates shear stress induced PGHS-2 expre-ssion and PGE2 synthesis in MLO-Y4 osteocyte-like cells. Ann Biomed Eng. 39:378–393. DOI: 10.1007/s10439-010-0156-6. PMID: 20820919.
Article37. Takiguchi T, Kobayashi M, Nagashima C, Yamaguchi A, Nishihara T, Hasegawa K. 1999; Effect of prostaglandin E2 on recombinant human bone morphogenetic protein-2-stimulated osteoblastic differentiation in human periodontal ligament cells. J Periodontal Res. 34:431–436. DOI: 10.1111/j.1600-0765.1999.tb02278.x. PMID: 10685373.38. Kikuta S, Tanaka N, Kazama T, Kazama M, Kano K, Ryu J, Tokuhashi Y, Matsumoto T. 2013; Osteogenic effects of dedifferentiated fat cell transplantation in rabbit models of bone defect and ovariectomy-induced osteoporosis. Tissue Eng Part A. 19:1792–1802. DOI: 10.1089/ten.tea.2012.0380. PMID: 23566022. PMCID: PMC3700015.
Article39. Tateno A, Asano M, Akita D, Toriumi T, Tsurumachi-Iwasaki N, Kazama T, Arai Y, Matsumoto T, Kano K, Honda M. 2019; Transplantation of dedifferentiated fat cells combined with a biodegradable type I collagen-recombinant peptide scaffold for critical-size bone defects in rats. J Oral Sci. 61:534–538. DOI: 10.2334/josnusd.18-0458. PMID: 31631097.
Article40. Shirakata Y, Nakamura T, Shinohara Y, Taniyama K, Sakoda K, Yoshimoto T, Noguchi K. 2014; An exploratory study on the efficacy of rat dedifferentiated fat cells (rDFATs) with a poly lactic-co-glycolic acid/hydroxylapatite (PLGA/HA) composite for bone formation in a rat calvarial defect model. J Mater Sci Mater Med. 25:899–908. DOI: 10.1007/s10856-013-5124-x. PMID: 24363067.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Wnt/b-Catenin Promotes the Osteoblastic Potential of BMP9 Through Down-Regulating Cyp26b1 in Mesenchymal Stem Cells
- LIPUS Promotes Endothelial Differentiation and Angiogenesis of Periodontal Ligament Stem Cells by Activating Piezo1
- Effects of Low Intensity Ultrasound on the Co-Culture of Human Osteoblastic Cells (SaOS-2) with Endothelial Cells (HUVEC)
- Effect on bone healing by the application of low intensity pulsed ultrasound after injection of adipose tissue-derived stem cells at the implantation of titanium implant in the tibia of diabetes-induced rat
- Nectandrin A Enhances the BMP-Induced Osteoblastic Differentiation and Mineralization by Activation of p38 MAPK-Smad Signaling Pathway