J Korean Med Sci.
2023 Nov;38(44):e346. 10.3346/jkms.2023.38.e346.
Adverse Drug Events Associated With Remdesivir in Real-World Hospitalized Patients With COVID-19, Including Vulnerable Populations: A Retrospective Multicenter Study
- Affiliations
-
- 1Infection Control Center, Seoul National University Hospital, Seoul, Korea
- 2College of Pharmacy, Seoul National University, Seoul, Korea
- 3Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 4Department of Internal Medicine, Inha University College of Medicine, Incheon, Korea
- 5Department of Pharmacy, Seoul National University Hospital, Seoul, Korea
- 6Department of Preventive Medicine, University of Ulsan College of Medicine, Ulsan University Hospital, Ulsan, Korea
- KMID: 2547969
- DOI: http://doi.org/10.3346/jkms.2023.38.e346
Abstract
- Background
Remdesivir is a US Food and Drug Administration-approved drug for coronavirus disease 2019 (COVID-19). Clinical trials were conducted under strictly controlled situations for a selected population, and their reported adverse events may not fully represent conditions in real-world patients. We aimed to estimate the incidence of adverse drug events (ADEs) associated with remdesivir in hospitalized patients with COVID-19, including vulnerable subpopulations, such as those with impaired renal or hepatic function and pregnant women.
Methods
This retrospective observational study included hospitalized patients with confirmed COVID-19 treated with remdesivir between January and December 2021 at ten hospitals. ADEs and severe ADEs (Common Toxicity Criteria for Adverse Events grade ≥ 3) were operationally defined and analyzed through laboratory investigations. The incidence of ADEs was compared with that of each matched control in subpopulations with renal or hepatic impairment and pregnant women.
Results
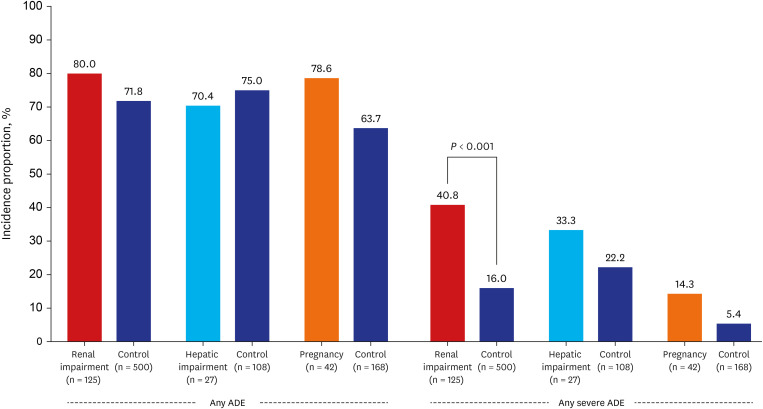
Among 2,140 patients, 1,416 (66.2%) and 295 (13.8%) experienced at least one ADE and severe ADE, respectively. The most frequent ADE was 'hepatic injury' (42.9%), followed by anemia (27.6%). The most common severe ADEs were 'hypokalemia' (5.3%), 'hepatic injury' (2.9%), and 'anemia' (3.6%). There was no significant difference in the incidence of ADEs in patients relative to their respective matched-control groups, including those with renal impairment (80.0% vs. control 71.8%, P = 0.063), hepatic impairment (70.4% vs. control 75.0%, P = 0.623) and pregnant women (78.6% vs. control 63.7%, P = 0.067). However, severe ADE incidence was significantly higher in patients with renal impairment (40.8% vs. 16.0%, P < 0.001). The most common severe ADEs in those were 'anemia' (15.3%), 'hypokalemia' (10.5%), and 'thrombocytopenia' (8.9%). There was no statistically significant difference in the incidence of severe ADEs in patients with hepatic impairment or in pregnancy (P = 0.230; P = 0.085).
Conclusion
A significant proportion of patients with COVID-19 treated with remdesivir experienced ADEs and severe ADEs. Given the high incidence of severe ADEs, caution is required in patients with renal impairment. Further studies are needed to investigate ADEs in pregnant women and patients with hepatic impairment.
Figure
Reference
-
1. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. Updated 2022. Accessed August 17, 2022. https://www.covid19treatmentguidelines.nih.gov/ .2. WHO coronavirus (COVID-19) dashboard. Updated 2020. Accessed November 30, 2022. https://covid19.who.int/ .3. Gilead Sciences Inc. VEKLURY® intravenous injection (package insert). Updated 2020. Accessed December 12, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214787Orig1s000lbl.pdf .4. Park DH, Kang CK, Choe PG, Kim NJ, Park WB, Oh MD. How we have treated severe to critically ill patients with coronavirus disease 2019 in Korea. J Korean Med Sci. 2022; 37(49):e353. PMID: 36536547.
Article5. Jeon J, Chin B. Treatment options for patients with mild-to-moderate coronavirus disease 2019 in Korea. J Korean Med Sci. 2022; 37(48):e352. PMID: 36513054.
Article6. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020; 383(19):1813–1826. PMID: 32445440.
Article7. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020; 395(10236):1569–1578. PMID: 32423584.8. Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020; 383(19):1827–1837. PMID: 32459919.
Article9. Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020; 324(11):1048–1057. PMID: 32821939.
Article10. Elsawah HK, Elsokary MA, Abdallah MS, ElShafie AH. Efficacy and safety of remdesivir in hospitalized COVID-19 patients: systematic review and meta-analysis including network meta-analysis. Rev Med Virol. 2021; 31(4):e2187. PMID: 33128490.
Article11. Lai CC, Chen CH, Wang CY, Chen KH, Wang YH, Hsueh PR. Clinical efficacy and safety of remdesivir in patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2021; 76(8):1962–1968. PMID: 33758946.
Article12. Charan J, Kaur RJ, Bhardwaj P, Haque M, Sharma P, Misra S, et al. Rapid review of suspected adverse drug events due to remdesivir in the WHO database; findings and implications. Expert Rev Clin Pharmacol. 2021; 14(1):95–103. PMID: 33252992.
Article13. Chiu MN, Bhardwaj M, Sah SP. Safety profile of COVID-19 drugs in a real clinical setting. Eur J Clin Pharmacol. 2022; 78(5):733–753. PMID: 35088108.
Article14. Fan Q, Zhang B, Ma J, Zhang S. Safety profile of the antiviral drug remdesivir: An update. Biomed Pharmacother. 2020; 130:110532. PMID: 32707440.
Article15. Veklury: EPAR - product information. Updated 2023. Accessed February 10, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/veklury#product-information-section .16. Guidance on the use of COVID-19 treatment. 10th ed. Updated 2022. Accessed December 12, 2022. https://ncv.kdca.go.kr/hcp/page.do?mid=030301 .17. Guidance on the use of COVID-19 treatment. 10-1th ed. Updated 2023. Accessed May 31, 2023. https://ncv.kdca.go.kr/hcp/page.do?mid=030301 .18. Estiverne C, Strohbehn IA, Mithani Z, Hirsch JS, Wanchoo R, Goyal PG, et al. Remdesivir in patients with estimated GFR <30 ml/min per 1.73 m2 or on renal replacement therapy. Kidney Int Rep. 2021; 6(3):835–838. PMID: 33263094.
Article19. Sunny S, Samaroo-Campbell J, Abdallah M, Luka A, Quale J. Is remdesivir safe in patients with renal impairment? Experience at a large tertiary urban medical center. Infection. 2023; 51(1):247–252. PMID: 35616879.
Article20. Sabers AJ, Williams AL, Farley TM. Use of remdesivir in the presence of elevated LFTs for the treatment of severe COVID-19 infection. BMJ Case Rep. 2020; 13(10):e239210.
Article21. Eastman RT, Roth JS, Brimacombe KR, Simeonov A, Shen M, Patnaik S, et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020; 6(5):672–683. PMID: 32483554.
Article22. Gupte V, Hegde R, Sawant S, Kalathingal K, Jadhav S, Malabade R, et al. Safety and clinical outcomes of remdesivir in hospitalised COVID-19 patients: a retrospective analysis of active surveillance database. BMC Infect Dis. 2022; 22(1):1. PMID: 34983406.
Article23. Bergamaschi G, Borrelli de Andreis F, Aronico N, Lenti MV, Barteselli C, Merli S, et al. Anemia in patients with COVID-19: pathogenesis and clinical significance. Clin Exp Med. 2021; 21(2):239–246. PMID: 33417082.
Article24. Thakare S, Gandhi C, Modi T, Bose S, Deb S, Saxena N, et al. Safety of remdesivir in patients with acute kidney injury or CKD. Kidney Int Rep. 2021; 6(1):206–210. PMID: 33073066.
Article25. Jdiaa SS, Mansour R, El Alayli A, Gautam A, Thomas P, Mustafa RA. COVID-19 and chronic kidney disease: an updated overview of reviews. J Nephrol. 2022; 35(1):69–85. PMID: 35013985.
Article26. Budi DS, Pratama NR, Wafa IA, Putra M, Wardhana MP, Wungu CD. Remdesivir for pregnancy: a systematic review of antiviral therapy for COVID-19. Heliyon (Lond). 2022; 8(1):e08835.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Systematic Review for Remdesivir Use in Pediatric Patients under 3.5 kg with COVID-19
- Analysis of Treatment-Related Adverse Events in Hospitalized Patients with Coronavirus Disease 2019
- Effectiveness of Regdanvimab and Remdesivir in Patients with COVID-19 at a Single Hospital
- A Case Report for Severe COVID-19 in a 9-Year-Old Child Treated with Remdesivir and Dexamethasone
- Adverse events following vaccination against coronavirus disease 2019