Healthc Inform Res.
2023 Oct;29(4):334-342. 10.4258/hir.2023.29.4.334.
Factors Influencing the Acceptance of Distributed Research Networks in Korea: Data Accessibility and Data Security Risk
- Affiliations
-
- 1College of Liberal Arts, Dankook University, Cheonan, Korea
- 2College of Health Science, Dankook University, Cheonan, Korea
- KMID: 2547686
- DOI: http://doi.org/10.4258/hir.2023.29.4.334
Abstract
Objectives
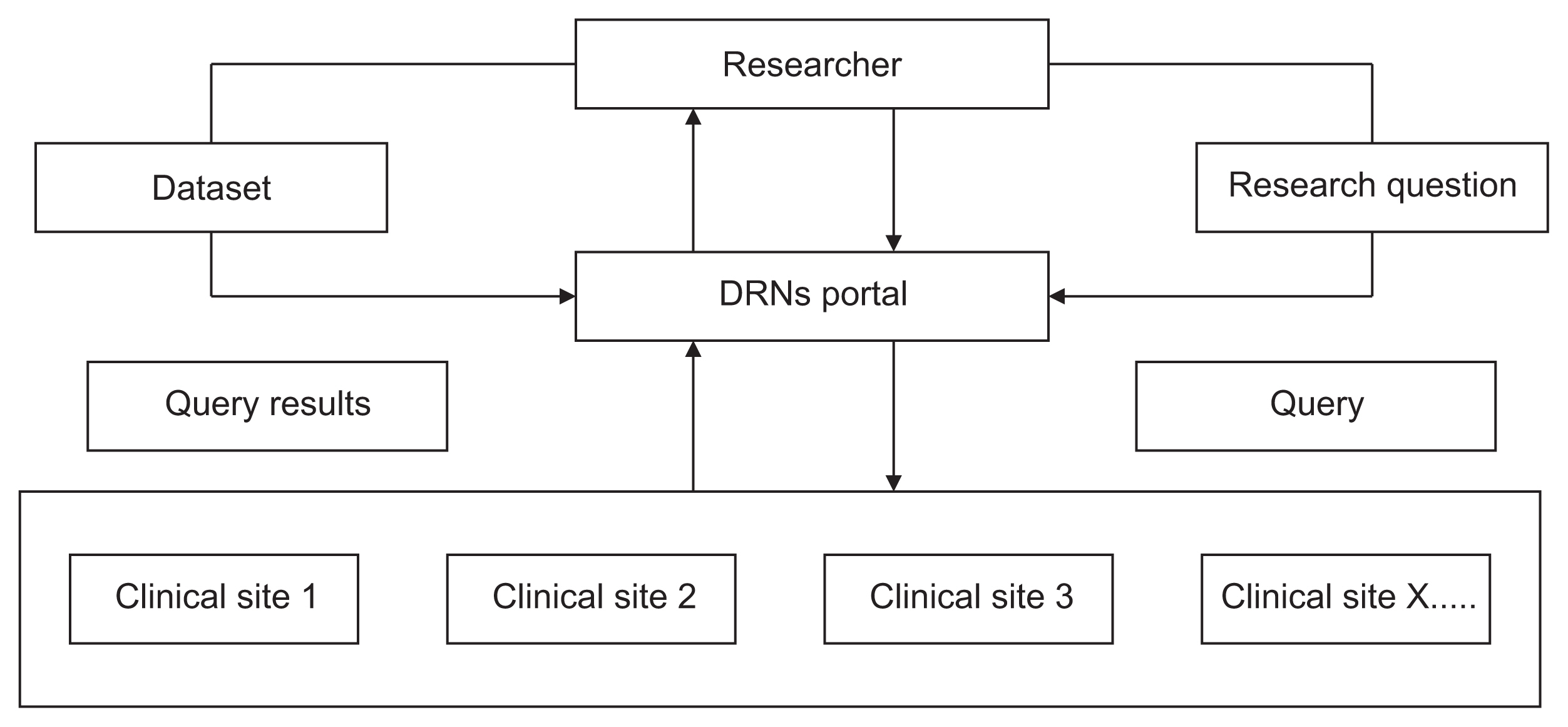
Distributed research networks (DRNs) facilitate multicenter research by enabling the use of multicenter data; therefore, they are increasingly utilized in healthcare fields. Despite the numerous advantages of DRNs, it is crucial to understand researchers' acceptance of these networks to ensure their effective application in multicenter research. In this study, we sought to identify the factors influencing the adoption of DRNs among researchers in Korea.
Methods
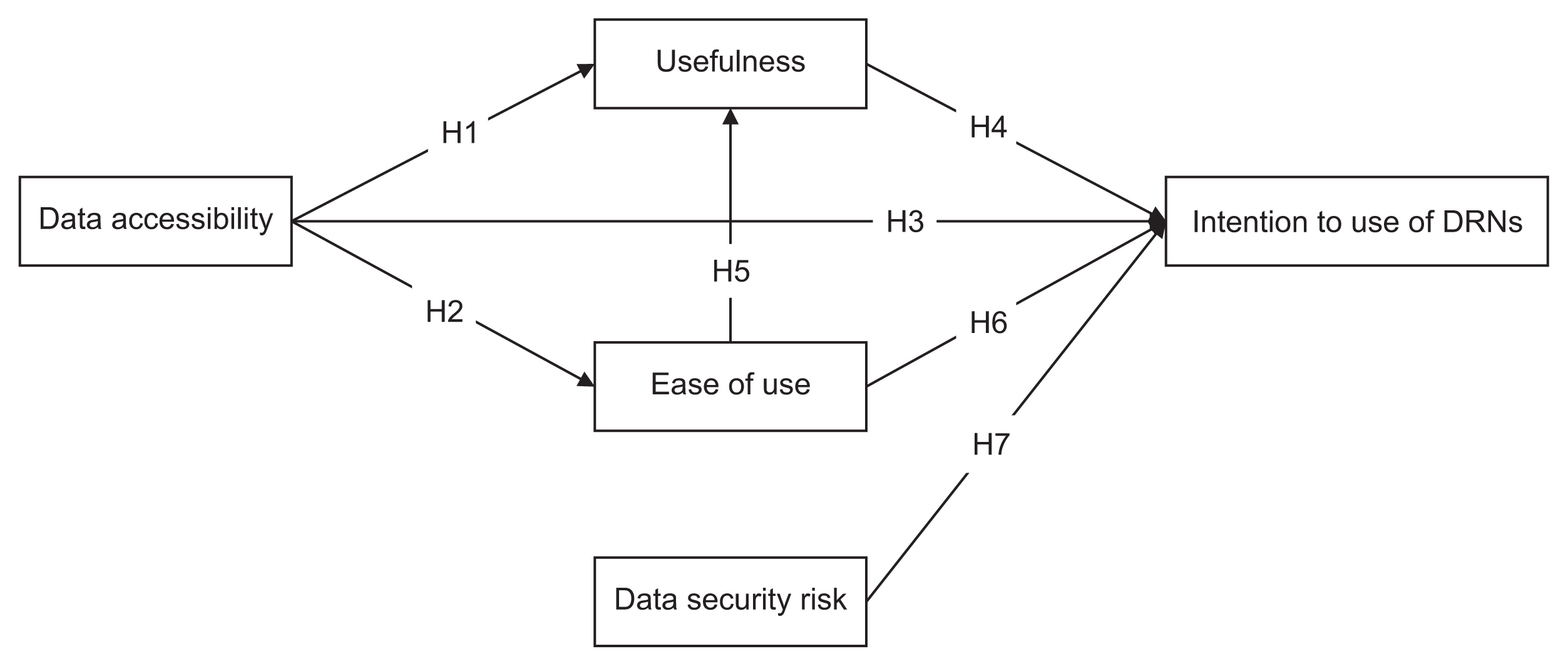
We used snowball sampling to collect data from 149 researchers between July 7 and August 28, 2020. Five factors were used to formulate the hypotheses and research model: data accessibility, usefulness, ease of use, data security risk, and intention to use DRNs. We applied a structural equation model to identify relationships within the research model.
Results
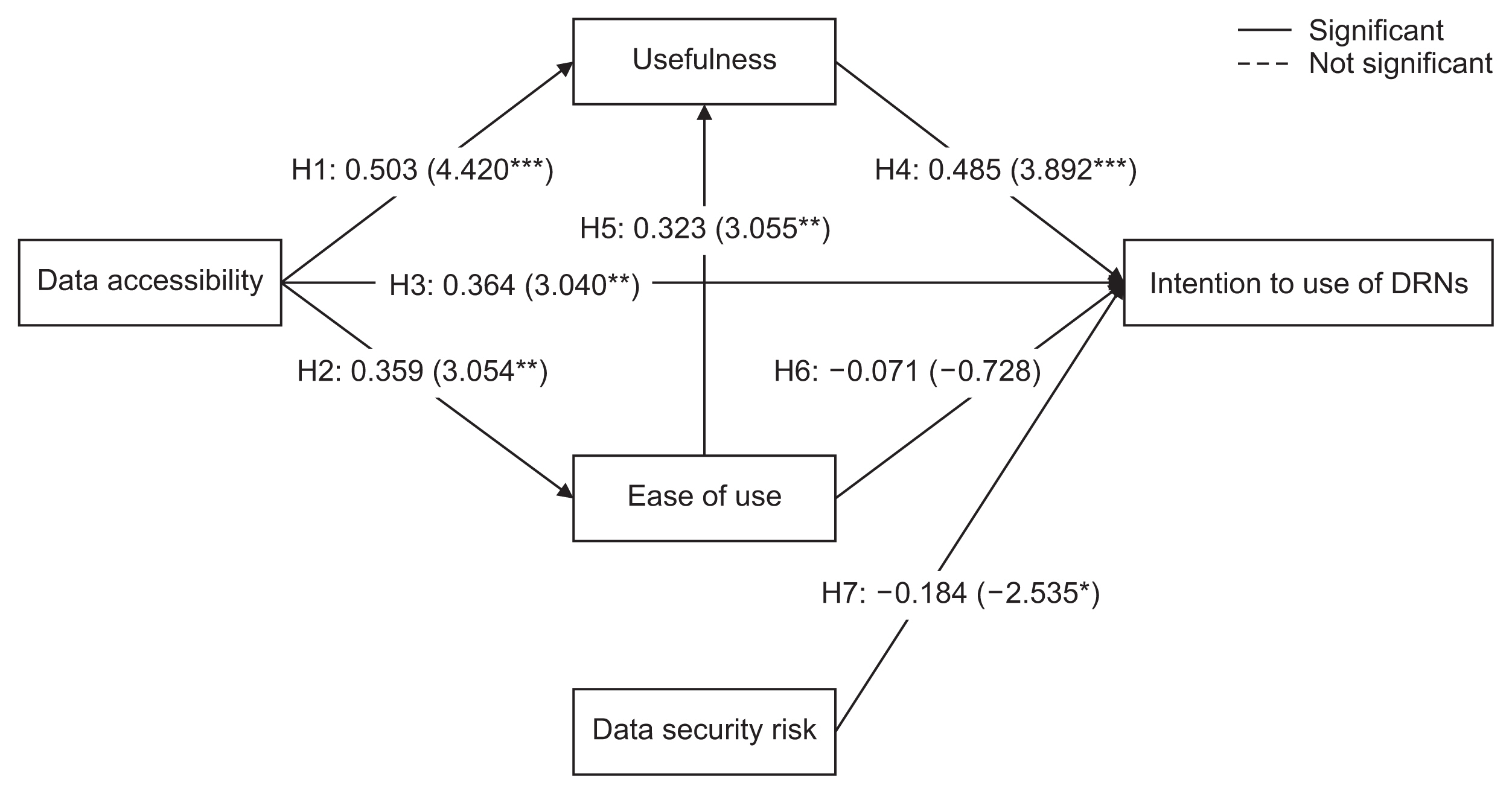
Data accessibility and data security were critical to the acceptance and use of DRNs. The usefulness of DRNs partially mediated the relationship between data accessibility and the intention to use DRNs. Interestingly, ease of use did not influence the intention to use DRNs, but it was affected by data accessibility. Furthermore, ease of use impacted the perceived usefulness of DRNs.
Conclusions
This study highlighted major factors that can promote the broader adoption and utilization of DRNs. Consequently, these findings can contribute to the expansion of active multicenter research using DRNs in the field of healthcare research.
Figure
Reference
-
References
1. Bredfeldt CE, Butani A, Padmanabhan S, Hitz P, Pardee R.Managing protected health information in distributed research network environments: automated review to facilitate collaboration. BMC Med Inform Decis Mak. 2013; 13:39. https://doi.org/10.1186/1472-6947-13-39.
Article2. Velentgas P, Bohn RL, Brown JS, Chan KA, Gladowski P, Holick CN, et al. A distributed research network model for post-marketing safety studies: the Meningococcal Vaccine Study. Pharmacoepidemiol Drug Saf. 2008; 17(12):1226–34. https://doi.org/10.1002/pds.1675.
Article3. Toh S, Platt R, Steiner JF, Brown JS.Comparative-effectiveness research in distributed health data networks. Clin Pharmacol Ther. 2011; 90(6):883–7. https://doi.org/10.1038/clpt.2011.236.
Article4. Holmes JH, Elliott TE, Brown JS, Raebel MA, Davidson A, Nelson AF, et al. Clinical research data warehouse governance for distributed research networks in the USA: a systematic review of the literature. J Am Med Inform Assoc. 2014; 21(4):730–6. https://doi.org/10.1136/amiajnl-2013-002370.
Article5. Forrow S, Campion DM, Herrinton LJ, Nair VP, Robb MA, Wilson M, et al. The organizational structure and governing principles of the Food and Drug Administration’s Mini-Sentinel pilot program. Pharmacoepidemiol Drug Saf. 2012; 21 (Suppl 1):12–7. https://doi.org/10.1002/pds.2242.
Article6. McMurry AJ, Gilbert CA, Reis BY, Chueh HC, Kohane IS, Mandl KD.A self-scaling, distributed information architecture for public health, research, and clinical care. J Am Med Inform Assoc. 2007; 14(4):527–33. https://doi.org/10.1197/jamia.M2371.
Article7. Korea Health Information Service. Health and medical big data platform [Internet]. Seoul, Korea: Korea Health Information Service;c2023. [cited at 2023 Sep 30]; Available from: https://hcdl.mohw.go.kr.8. Korea Institute of Drug Safety & Risk Management. MOA project: medical record observation and assessment for drug safety [Internet]. Anyang, Korea: Korea Institute of Drug Safety & Risk Management;c2023. [cited at 2023 Sep 30]. Available from: https://moa.drug-safe.or.kr/main.9. Venkatesh V, Morris MG, Davis GB, Davis FD.User acceptance of information technology: toward a unified view. MIS Q. 2003; 27(3):425–78. https://doi.org/10.2307/30036540.
Article10. Sandberg J, Trief PM, Izquierdo R, Goland R, Morin PC, Palmas W, et al. A qualitative study of the experiences and satisfaction of direct telemedicine providers in diabetes case management. Telemed J E Health. 2009; 15(8):742–50. https://doi.org/10.1089/tmj.2009.0027.
Article11. Dunn HP, Kang CJ, Marks S, Witherow JL, Dunn SM, Healey PR, et al. Perceived usefulness and ease of use of fundoscopy by medical students: a randomised cross-over trial of six technologies (eFOCUS 1). BMC Med Educ. 2021; 21(1):41. https://doi.org/10.1186/s12909-020-02469-8.
Article12. Schnall R, Higgins T, Brown W, Carballo-Dieguez A, Bakken S.Trust, perceived risk, perceived ease of use and perceived usefulness as factors related to mHealth technology use. Stud Health Technol Inform. 2015; 216:467–71.13. Rho MJ, Kim HS, Chung K, Choi IY.Factors influencing the acceptance of telemedicine for diabetes management. Cluster Comput. 2015; 18(1):321–31. https://doi.org/10.1007/s10586-014-0356-1.
Article14. Venkatesh V, Davis FD.A theoretical extension of the technology acceptance model: four longitudinal field studies. Manag Sci. 2000; 46(2):186–204. https://doi.org/10.1287/mnsc.46.2.186.11926.
Article15. Rho MJ, Choi IY, Lee J.Predictive factors of telemedicine service acceptance and behavioral intention of physicians. Int J Med Inform. 2014; 83(8):559–71. https://doi.org/10.1016/j.ijmedinf.2014.05.005.
Article16. Kamal SA, Shafiq M, Kakria P.Investigating acceptance of telemedicine services through an extended technology acceptance model (TAM). Technol Soc. 2020; 60:101212. https://doi.org/10.1016/j.techsoc.2019.101212.
Article17. de Sena Abrahao R, Moriguchi SN, Andrade DF.Intention of adoption of mobile payment: an analysis in the light of the Unified Theory of Acceptance and Use of Technology (UTAUT). RAI Revista de Administracaoe Inovacao. 2016; 13(3):221–30. https://doi.org/10.1016/j.rai.2016.06.003.
Article18. Sangroya A, Kumar S, Dhok J, Varma V. Towards analyzing data security risks in cloud computing environments. Prasad SK, Vin HM, Sahni S, Jaiswal MP, Thipakorn B, editors. Information systems, technology and management. Heidelberg, Germany: Springer;2010. 255–65. https://doi.org/10.1007/978-3-642-12035-0_25.
Article19. Kim KK, McGraw D, Mamo L, Ohno-Machado L.Development of a privacy and security policy framework for a multistate comparative effectiveness research network. Med Care. 2013; 51(8 Suppl 3):S66–72. https://doi.org/10.1097/MLR.0b013e31829b1d9f.
Article20. Goodman LA.Snowball sampling. Ann Math Stat. 1961; 32(3):148–70.
Article21. Parker C, Scott S, Geddes A. Snowball sampling. Thousand Oaks (CA): SAGE Publications Ltd;2019. https://doi.org/10.4135/9781526421036831710.
Article22. Rosseel Y, Jorgensen TD, Rockwood N, Oberski D, Byrnes J, Vanbrabant L, et al. Package ‘lavaan’: latent variable analysis [Internet]. Vienna, Austria: The R Foundation;2023. [cited at 2023 Sep 30]. Available from: https://cran.r-project.org/web/packages/lavaan/lavaan.pdf.23. Nunnally JC. Psychometric theory. 2nd ed. New York (NY): McGraw-Hill;1978.24. Chin WW. The partial least squares approach to structural equation modeling. Marcoulides GA, editor. Modern methods for business research. Mahwah (NJ): Lawrence Erlbaum Associates;1998. p. 295–336.25. Hair JF. Multivariate data analysis. 7th ed. Upper Saddle River (NJ): Prentice Hall;2010.26. Curtis LH, Weiner MG, Boudreau DM, Cooper WO, Daniel GW, Nair VP, et al. Design considerations, architecture, and use of the Mini-Sentinel distributed data system. Pharmacoepidemiol Drug Saf. 2012; 21 (Suppl 1):23–31. https://doi.org/10.1002/pds.2336.
Article27. Nadri H, Rahimi B, Lotfnezhad Afshar H, Samadbeik M, Garavand A.Factors affecting acceptance of hospital information systems based on extended technology acceptance model: a case study in three paraclinical departments. Appl Clin Inform. 2018; 9(2):238–47. https://doi.org/10.1055/s-0038-1641595.
Article28. Abdekhoda M, Ahmadi M, Gohari M, Noruzi A.The effects of organizational contextual factors on physicians’ attitude toward adoption of Electronic Medical Records. J Biomed Inform. 2015; 53:174–9. https://doi.org/10.1016/j.jbi.2014.10.008.
Article29. Hsiao JL, Chang HC, Chen RF.A study of factors affecting acceptance of hospital information systems: a nursing perspective. J Nurs Res. 2011; 19(2):150–60. https://doi.org/10.1097/JNR.0b013e31821cbb25.
Article30. Guo XT, Yuan JQ, Cao XF, Chen XD. Understanding the acceptance of mobile health services: a service participants analysis. In : Proceedings of 2012 International Conference on Management Science & Engineering (ICMSE) 19th Annual Conference; 2012 Sep 2022; Dallas, TX. p. 1868–73. https://doi.org/10.1109/ICMSE.2012.6414426.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Factors Influencing the Adoption of Advanced Cryptographic Techniques for Data Protection of Patient Medical Records
- Ethics for Artificial Intelligence: Focus on the Use of Radiology Images
- Transaction Serializability with Security in Heterogeneous Medical Database Systems
- Web-based Secure Access from Multiple Patient Reservoirs
- Investigating the Relationship Between Accessibility of Green Space and Adult Obesity Rates: A Secondary Data Analysis in the United States