Brain Tumor Res Treat.
2023 Oct;11(4):232-238. 10.14791/btrt.2023.0021.
A Case Series of DuraMatrix-Onlay® Plus in Cranial Surgery Is Associated With a Low Complication Profile
- Affiliations
-
- 1Departments of Neurosurgery, University of California, Los Angeles, Los Angeles, CA, USA
- 2Departments of Radiation Oncology, Surgery, University of California, Los Angeles, Los Angeles, CA, USA
- 3Departments of Head and Neck Surgery, University of California, Los Angeles, Los Angeles, CA, USA
- 4Jonsson Comprehensive Cancer Center, Ronald Reagan UCLA Medical Center of the David Geffen School of Medicine at the University of California, Los Angeles, Los Angeles, CA, USA
- 5Department of Neurosurgery, Harbor-UCLA Medical Center, Torrance, CA, USA
- 6Los Angeles Biomedical Research Institute (LA BioMed), Harbor-UCLA Medical Center, Torrance, CA, USA
- KMID: 2547411
- DOI: http://doi.org/10.14791/btrt.2023.0021
Abstract
- Background
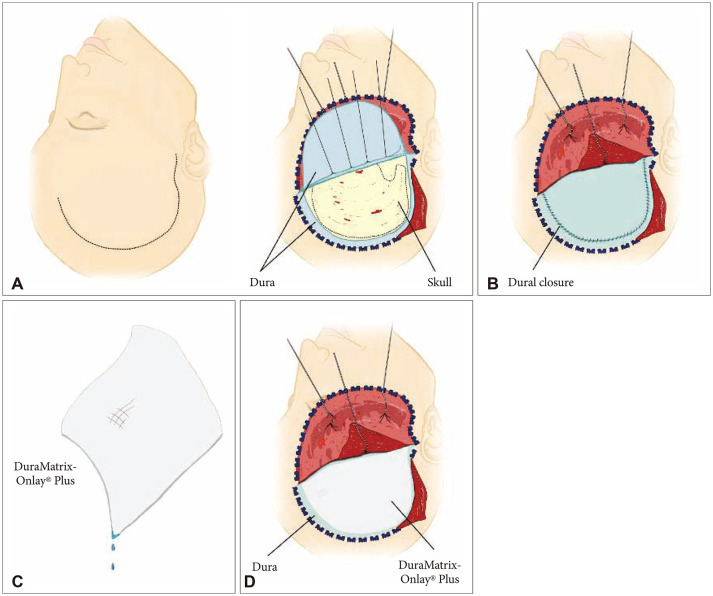
DuraMatrix-Onlay® Plus is a collagen dura membrane derived from purified bovine Achilles tendon. The matrix provides a scaffold for collagen synthesis and is intended to be used as an onlay without the need for dural sutures. The study aims to describe our experience with 33 consecutive patients who underwent a duraplasty procedure using the novel DuraMatrix-Onlay® Plus collagen dura membrane.
Methods
This is a retrospective case series of 33 patients who underwent a duraplasty proce- dure at a single academic hospital in Los Angeles, CA, USA between May 2016 and March 2017. The primary outcome was the incidence rate of cerebrospinal fluid (CSF) leak. Secondary outcomes included rates of patient infection, dural substitute complication, and removal.
Results
Thirty-three patients underwent a duraplasty procedure using the DuraMatrix-Onlay® Plus material. The average age of the patients was 41.12±7.34 years (range 2–75 years). There were 18 (54.5%) females and 15 (45.5%) males. The majority of procedures were elective operations for the resection of a lesion (n=19, 58%), and the average graft size was 17.69±4.73 cm2 . At an average follow-up of 3 months, there were no postoperative CSF leaks. The rates of patient infection, dural substitute complication, and removal were 6%, 6%, and 3%, respectively.
Conclusion
DuraMatrix-Onlay® Plus is associated with a low rate of postoperative CSF leakage and an acceptable complication profile. This result supports the use of collagen matrices for dural closure in general neurosurgical procedures.
Keyword
Figure
Reference
-
1. Caroli E, Rocchi G, Salvati M, Delfini R. Duraplasty: our current experience. Surg Neurol. 2004; 61:55–59. discussion 59. PMID: 14706380.
Article2. Barrientos S, Leif M, Hon HH, Aizenberg M, Wong S. Duraplasty using autologous fascia lata and latissimus dorsi free flap for chronic cerebrospinal fluid leak. J Craniofac Surg. 2019; 30:e671–e674. PMID: 31574789.
Article3. Choi EH, Chan AY, Brown NJ, Lien BV, Sahyouni R, Chan AK, et al. Effectiveness of repair techniques for spinal dural tears: a systematic review. World Neurosurg. 2021; 149:140–147. PMID: 33640528.
Article4. Narotam PK, van Dellen JR, Bhoola KD. A clinicopathological study of collagen sponge as a dural graft in neurosurgery. J Neurosurg. 1995; 82:406–412. PMID: 7861218.
Article5. Lee JF, Odom GL, Tindall GT. Experimental evalation of silicone-coated dacron and collagen fabric-film laminate as dural substitutes. J Neurosurg. 1967; 27:558–564. PMID: 6080098.
Article6. van der Veen VC, van der Wal MB, van Leeuwen MC, Ulrich MM, Middelkoop E. Biological background of dermal substitutes. Burns. 2010; 36:305–321. PMID: 19897310.
Article7. Ralston DR, Layton C, Dalley AJ, Boyce SG, Freedlander E, Mac Neil S. The requirement for basement membrane antigens in the production of human epidermal/dermal composites in vitro. Br J Dermatol. 1999; 140:605–615. PMID: 10233309.
Article8. Narotam PK, Reddy K, Fewer D, Qiao F, Nathoo N. Collagen matrix duraplasty for cranial and spinal surgery: a clinical and imaging study. J Neurosurg. 2007; 106:45–51. PMID: 17236486.
Article9. Esposito F, Cappabianca P, Fusco M, Cavallo LM, Bani GG, Biroli F, et al. Collagen-only biomatrix as a novel dural substitute. Examination of the efficacy, safety and outcome: clinical experience on a series of 208 patients. Clin Neurol Neurosurg. 2008; 110:343–351. PMID: 18242823.10. Kshettry VR, Lobo B, Lim J, Sade B, Oya S, Lee JH. Evaluation of non-watertight dural reconstruction with collagen matrix onlay graft in posterior fossa surgery. J Korean Neurosurg Soc. 2016; 59:52–57. PMID: 26885286.
Article11. Stryker. DuraMatrix-Onlay Plus. Kalamazoo, MI: Stryker;Accessed August 13, 2023. at https://cmf.stryker.com/products/duramatrix-onlay-plus.12. Biroli F, Esposito F, Fusco M, Bani GG, Signorelli A, de Divitiis O, et al. Novel equine collagen-only dural substitute. Neurosurgery. 2008; 62(3 Suppl 1):273–274. discussion 274. PMID: 18424997.
Article13. Esposito F, Grimod G, Cavallo LM, Lanterna L, Biroli F, Cappabianca P. Collagen-only biomatrix as dural substitute: what happened after a 5-year observational follow-up study. Clin Neurol Neurosurg. 2013; 115:1735–1737. PMID: 23622936.
Article14. Azzam D, Romiyo P, Nguyen T, Sheppard JP, Alkhalid Y, Lagman C, et al. Dural repair in cranial surgery is associated with moderate rates of complications with both autologous and nonautologous dural substitutes. World Neurosurg. 2018; 113:244–248. PMID: 29374609.
Article15. Bolly HMB, Faried A, Jembise TL, Wirakusumah FF, Arifin MZ. The ideal selection criteria for duraplasty material in brain surgery: a review. Interdiscip Neurosurg. 2020; 22:100800.16. Wang W, Ao Q. Research and application progress on dural substitutes. J Neurorestoratology. 2019; 7:161–170.
Article17. Danish SF, Samdani A, Hanna A, Storm P, Sutton L. Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations. J Neurosurg. 2006; 104(1 Suppl):16–20. PMID: 16509475.
Article18. Strickland BA, Lucas J, Harris B, Kulubya E, Bakhsheshian J, Liu C, et al. Identification and repair of intraoperative cerebrospinal fluid leaks in endonasal transsphenoidal pituitary surgery: surgical experience in a series of 1002 patients. J Neurosurg. 2017; 129:425–429. PMID: 28960156.
Article19. Tabaee A, Anand VK, Barrón Y, Hiltzik DH, Brown SM, Kacker A, et al. Endoscopic pituitary surgery: a systematic review and meta-analysis. J Neurosurg. 2009; 111:545–554. PMID: 19199461.
Article20. Durham SR, Fjeld-Olenec K. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation type I in pediatric patients: a meta-analysis. J Neurosurg Pediatr. 2008; 2:42–49. PMID: 18590394.
Article21. Bowers CA, Brimley C, Cole C, Gluf W, Schmidt RH. AlloDerm for duraplasty in Chiari malformation: superior outcomes. Acta Neurochir (Wien). 2015; 157:507–511. PMID: 25377384.
Article22. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus. 2009; 26:E9.
Article23. Lee L, Ker J, Quah BL, Chou N, Choy D, Yeo TT. A retrospective analysis and review of an institution’s experience with the complications of cranioplasty. Br J Neurosurg. 2013; 27:629–635. PMID: 23879443.
Article24. Oladunjoye AO, Schrot RJ, Zwienenberg-Lee M, Muizelaar JP, Shahlaie K. Decompressive craniectomy using gelatin film and future bone flap replacement. J Neurosurg. 2013; 118:776–782. PMID: 23394343.
Article25. Raghavan A, Wright JM, Huang Wright C, Sajatovic M, Miller J. Effect of dural substitute and technique on cranioplasty operative metrics: a systematic literature review. World Neurosurg. 2018; 119:282–289. PMID: 30114536.
Article26. Sun H, Wang H, Diao Y, Tu Y, Li X, Zhao W, et al. Large retrospective study of artificial dura substitute in patients with traumatic brain injury undergo decompressive craniectomy. Brain Behav. 2018; 8:e00907. PMID: 29761002.
Article27. Pierson M, Birinyi PV, Bhimireddy S, Coppens JR. Analysis of decompressive craniectomies with subsequent cranioplasties in the presence of collagen matrix dural substitute and polytetrafluoroethylene as an adhesion preventative material. World Neurosurg. 2016; 86:153–160. PMID: 26433096.
Article28. Lee CH, Cho DS, Jin SC, Kim SH, Park DB. Usefulness of silicone elastomer sheet as another option of adhesion preventive material during craniectomies. Clin Neurol Neurosurg. 2007; 109:667–671. PMID: 17640798.
Article29. Costa BS, Cavalcanti-Mendes Gde A, de Abreu MS, de Sousa AA. Clinical experience with a novel bovine collagen dura mater substitute. Asian J Neurosurg. 2010; 5:31–34. PMID: 22028756.
Article30. Narotam PK, Qiao F, Nathoo N. Collagen matrix duraplasty for posterior fossa surgery: evaluation of surgical technique in 52 adult patients. Clinical article. J Neurosurg. 2009; 111:380–386. PMID: 19199453.
Article31. Cappabianca P, de Divitiis E. Endoscopic endonasal transsphenoidal surgery. Powell MP, Lightman SL, Laws ER, editors. Management of pituitary tumors. Totowa, NJ: Humana Press;2003. p. 161–171.32. Anson JA, Marchand EP. Bovine pericardium for dural grafts: clinical results in 35 patients. Neurosurgery. 1996; 39:764–768. PMID: 8880771.
Article33. Spake C, Beqiri D, Rao V, Crozier JW, Svokos KA, Woo AS. Subgaleal drains offer protection against infection in autologous cranioplasty, regardless of defect size. Plast Reconstr Surg Glob Open. 2021; 9(10 Suppl):49.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Surgical Outcome between Onlay Island Flap and Tubularized Incised Plate Urethroplasty in Hypospadias Repair
- Proximal Femur Salvage in Revision Knee Arthroplasty Due to Oncologic Indications: Long-term Results of Onlay and Overlapping Allograft in Revision Surgeries
- Onlay Island Flap Hypospadias Repair ; Experience of 45 Cases
- A Guide to Designing a Case Series in Plastic Surgery
- Treatment of difficult Nonunion by Modified Dual Onlay graft