Ann Clin Neurophysiol.
2023 Oct;25(2):106-109. 10.14253/acn.2023.25.2.106.
Inclusion body myositis accompanied with T-cell large granular lymphocyte leukemia
- Affiliations
-
- 1Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Plastic and Reconstructive Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Hematology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Department of Neurology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2547403
- DOI: http://doi.org/10.14253/acn.2023.25.2.106
Abstract
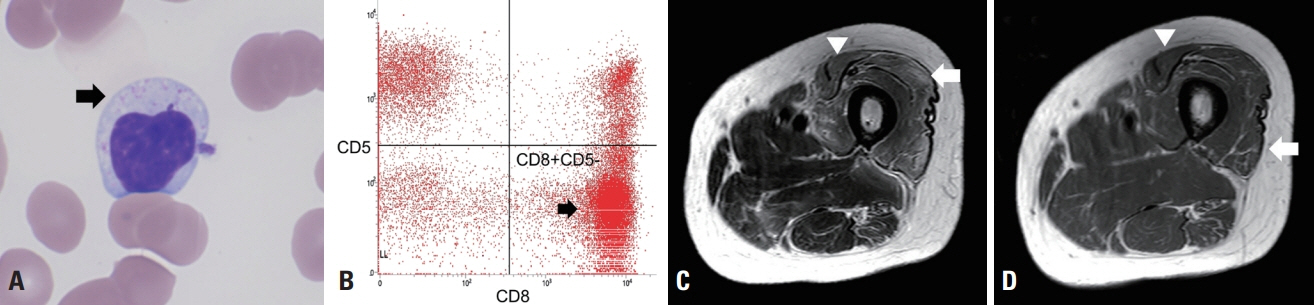
- Inclusion body myositis (IBM) is a late-onset myopathy that manifests as distinct muscle weakness in the quadriceps, finger flexors, and ankle dorsiflexors. T-cell large granular lymphocyte ( T-LGL) leukemia is a late-onset clonal disorder of CD8+ cy totoxic T-cells that is of ten accompanied by autoimmune diseases. To date, the association between IBM and T-LGL leukemia has been infrequently reported. Here, we report a case of a patient with T-LGL leukemia who developed IBM, along with in-depth laboratory, electrophysiological, and pathologic findings.
Figure
Reference
-
1. Rose MR. 188th ENMC international workshop: inclusion body myositis, 2-4 December 2011, Naarden, The Netherlands. Neuromuscul Disord. 2013; 23:1044–1055.
Article2. Lloyd TE, Mammen AL, Amato AA, Weiss MD, Needham M, Greenberg SA. Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology. 2014; 83:426–433.
Article3. Engel AG, Arahata K. Monoclonal antibody analysis of mononuclear cells in myopathies. II: Phenotypes of autoinvasive cells in polymyositis and inclusion body myositis. Ann Neurol. 1984; 16:209–215.
Article4. Mastaglia FL, Needham M, Scott A, James I, Zilko P, Day T, et al. Sporadic inclusion body myositis: HLA-DRB1 allele interactions influence disease risk and clinical phenotype. Neuromuscul Disord. 2009; 19:763–765.
Article5. Larman HB, Salajegheh M, Nazareno R, Lam T, Sauld J, Steen H, et al. Cytosolic 5’-nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann Neurol. 2013; 73:408–418.
Article6. Arnardottir S, Ansved T, Nennesmo I, Borg K. Report of a patient with inclusion body myositis and CD8+ chronic lymphocytic leukaemia--post-mortem analysis of muscle and brain. Acta Neurol Scand. 2001; 103:131–135.
Article7. Greenberg SA, Pinkus JL, Amato AA, Kristensen T, Dorfman DM. Association of inclusion body myositis with T cell large granular lymphocytic leukaemia. Brain. 2016; 139:1348–1360.
Article8. Greenberg SA, Pinkus JL, Kong SW, Baecher-Allan C, Amato AA, Dorfman DM. Highly differentiated cytotoxic T cells in inclusion body myositis. Brain. 2019; 142:2590–2604.
Article9. Greenberg SA. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol. 2019; 15:257–272.
Article10. Askanas V, Engel WK, Nogalska A. Sporadic inclusion-body myositis: a degenerative muscle disease associated with aging, impaired muscle protein homeostasis and abnormal mitophagy. Biochim Biophys Acta. 2015; 1852:633–643.
Article