Anesth Pain Med.
2023 Jul;18(3):220-232. 10.17085/apm.22260.
Current clinical application of dantrolene sodium
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Daejeon Eulji Medical Center, Eulji University, School of Medicine, Daejeon, Korea
- 2Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Anesthesiology and Pain Medicine, Dongguk University Ilsan Hospital, Dongguk University, Goyang, Korea
- 4Department of Anesthesiology and Pain Medicine, Konyang University Hopsital, Konyang University, College of Medicine, Daejeon, Korea
- 5Department of Anesthesiology and Pain Medicine, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 6Department of Anesthesia, Analgesia and lntensive Care lVedicine, Bangabandhu Sheikh Mujib Medical University Dhaka, Bangladesh
- KMID: 2547030
- DOI: http://doi.org/10.17085/apm.22260
Abstract
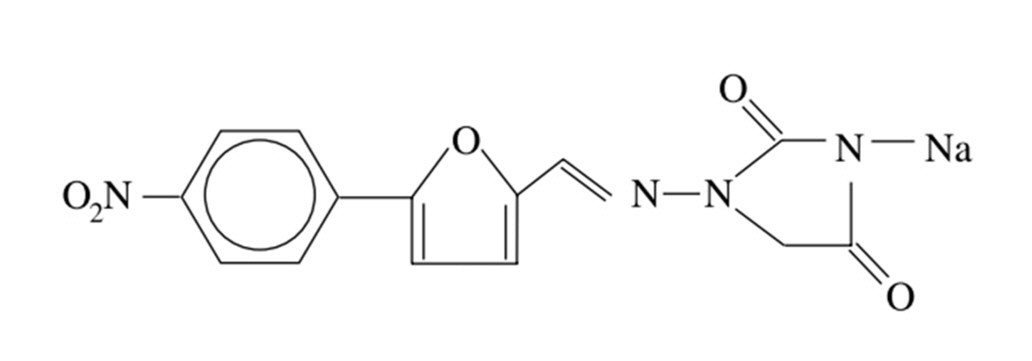
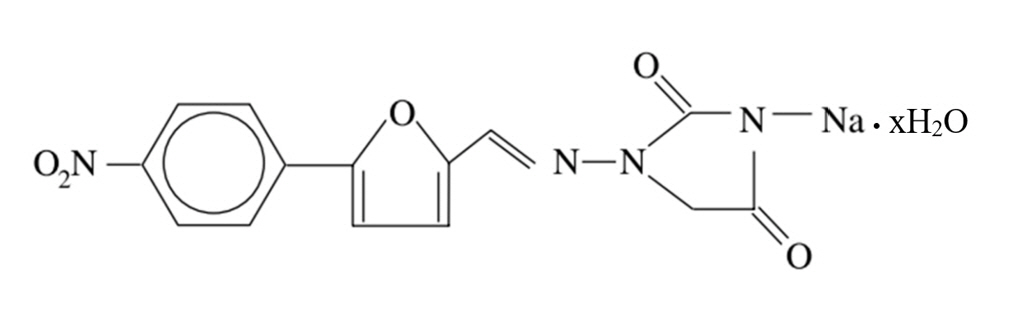
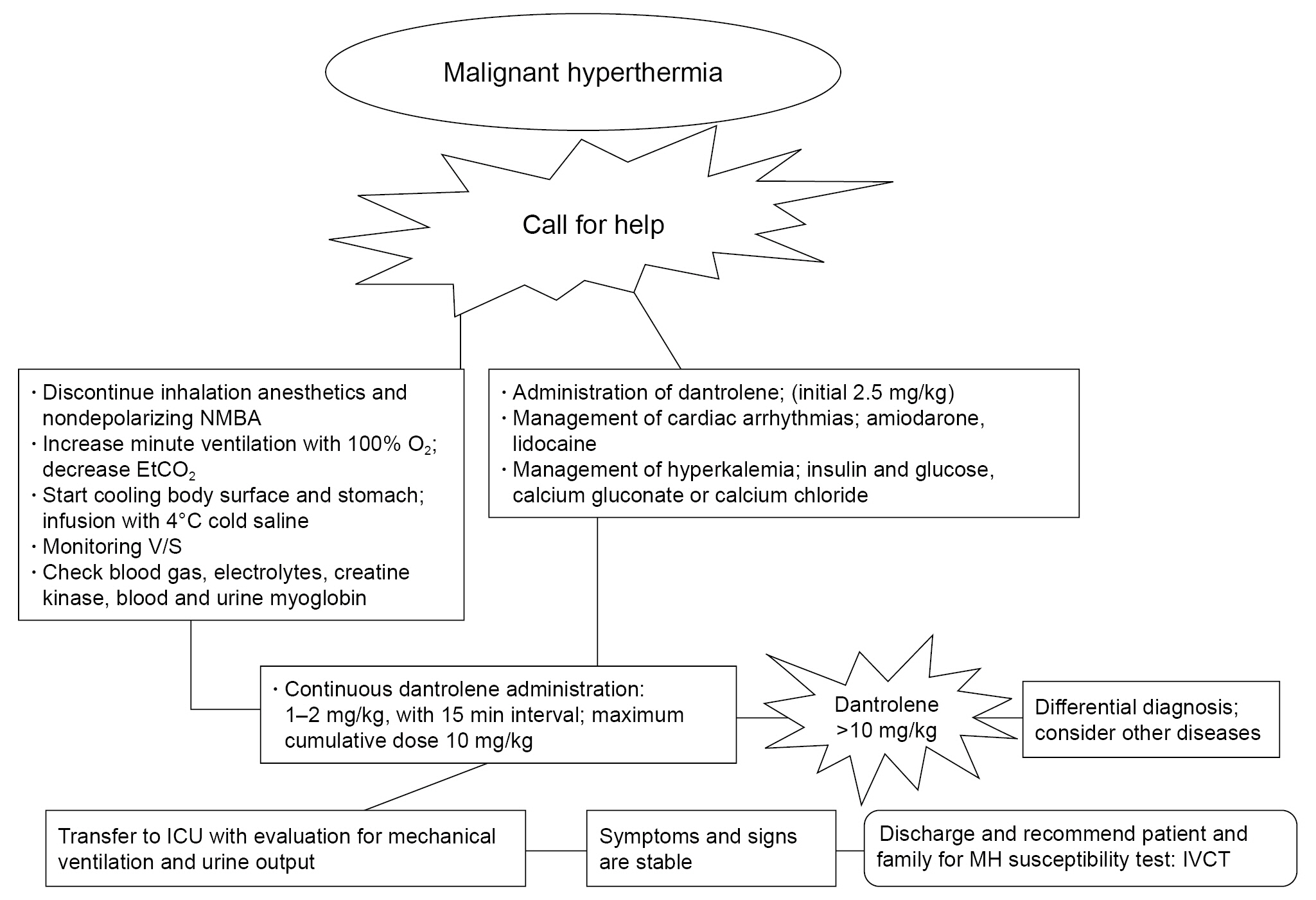
- Dantrolene sodium (DS) was first introduced as an oral antispasmodic drug. However, in 1975, DS was demonstrated to be effective for managing malignant hyperthermia (MH) and was adopted as the primary therapeutic drug after intravenous administration. However, it is difficult to administer DS intravenously to manage MH. MH is life-threatening, pharmacogenomically related, and induced by depolarizing neuromuscular blocking agents or inhalational anesthetics. All anesthesiologists should know the pharmacology of DS. DS suppresses Ca2+ release from ryanodine receptors (RyRs). RyRs are expressed in various tissues, although their distribution differs among subtypes. The anatomical and physiological functions of RyRs have also been demonstrated as effective therapeutic drugs for cardiac arrhythmias, Alzheimer’s disease, and other RyR-related diseases. Recently, a new formulation was introduced that enhanced the hydrophilicity of the lipophilic DS. The authors summarize the pharmacological properties of DS and comment on its indications, contraindications, adverse effects, and interactions with other drugs by reviewing reference articles.
Keyword
Figure
Reference
-
1. Denborough MA, Forster JFA, Lovell RRH, Maplestone PA, Villiers JD. Anaesthetic deaths in a family. Br J Anaesth. 1962; 34:395–6.
Article2. Nelson TE. Malignant hyperthermia: a pharmacogenetic disease of Ca++ regulating proteins. Curr Mol Med. 2002; 2:347–69.
Article3. Riazi S, Larach MG, Hu C, Wijeysundera D, Massey C, Kraeva N. Malignant hyperthermia in Canada: characteristics of index anesthetics in 129 malignant hyperthermia susceptible probands. Anesth Analg. 2014; 118:381–7.4. Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015; 10:93.
Article5. Inan S, Wei H. The cytoprotective effects of dantrolene: a ryanodine receptor antagonist. Anesth Analg. 2010; 111:1400–10.6. Jin W, Tan X, Wen J, Meng Y, Zhang Y, Li H, et al. A novel dantrolene sodium-loaded mixed micelle containing a small amount of cremophor EL: characterization, stability, safety and pharmacokinetics. Molecules. 2019; 24:728.
Article7. Pollock NA, Roslyn G, Machon RG, Rosenberg H. Early development, identification of mode of action, and use of dantrolene sodium: The role of Keith Ellis, Ph.D. Anesthesiology. 2017; 126:774–9.8. Litman RS, Rosenberg H. Malignant hyperthermia: update on susceptibility testing. JAMA. 2005; 293:2918–24.9. Hadad E, Cohen-Sivan Y, Heled Y, Epstein Y. Clinical review: treatment of heat stroke: should dantrolene be considered? Crit Care. 2005; 9:86–91.10. Grunau BE, Wiens MO, Brubacher JR. Dantrolene in the treatment of MDMA-related hyperpyrexia: a systematic review. CJEM. 2010; 12:435–42.
Article11. Harrison GG. Control of the malignant hyperpyrexic syndrome in MHS swine by dantrolene sodium. Br J Anaesth. 1975; 47:62–5.
Article12. Kim HJ, Koh WU, Choi JM, Ro YJ, Yang HS. Malignant hyperthermia and dantrolene sodium. Korean J Anesthesiol. 2019; 72:78–9.
Article13. Yip WH, Mingi CL, Ooi SJ, Chen SC, Chiang YY. A survey for prevention and treatment of malignant hyperthermia in Taiwan. Acta Anaesthesiol Taiwan. 2004; 42:147–51.14. Pfenninger E, Heiderich S, Klingler W. [Stocks of dantrolene in anesthesia and intensive care units in Germany: nationwide online survey with 1673 participants]. Anaesth. 2017; 66:773–81. German.15. McAvoy JC, Brodsky JB, Brock-Utne J. Pennywise and a Pound foolish: the advantage of dantrolene nanosuspension (Ryanodex) in the treatment of malignant hyperthermia. Anesth Analg. 2019; 129:e201–2.
Article16. Liang L, Wei H. Dantrolene, a treatment for Alzheimer disease? Alzheimer Dis Assoc Disord. 2015; 29:1–5.
Article17. Acsai K, Nagy N, Marton Z, Oravecz K, Varro A. Antiarrhythmic potential of drugs targeting the cardiac ryanodine receptor Ca2+ release channel: case study of dantrolene. Curr Pharm Des. 2015; 21:1062–72.18. Krause T, Gerbershagen MU, Fiege M, Weißhorn R, Wappler F. Dantrolene–a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004; 59:364–73.
Article19. Dantrium® Intravenous (dantrolene sodium for injection). Par Pharmaceutical, Inc. [Internet]. [updated 2017 Nov; cited 2023 Feb 20]. Available from https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=4df35098-8702-46be-ac67-30cfdf1aa570&type=display.20. Revonto® (dantrolene sodium for injection). USWM [Internet]. [updated 2020; cited 2023 Feb 20]. Available from https://revonto.com/wp-content/uploads/2021/10/revonto_pi.pdf.21. Revonto® (dantrolene sodium for injection). USWM [Internet]. [updated 2023; cited 2023 Feb 20]. Available from https://revonto.com/using-revonto/clinical-pharmacology/.22. Schütte JK, Becker S, Burmester S, Starosse A, Lenz D, Kröner L, et al. Comparison of the therapeutic effectiveness of a dantrolene sodium solution and a novel nanocrystalline suspension of dantrolene sodium in malignant hyperthermia normal and susceptible pigs. Eur J Anaesthesiol. 2011; 28:256–64.
Article23. Just KS, Gerbershagen MU, Grensemann J, Wappler F. Do we foresee new emerging drugs to treat malignant hyperthermia? Expert Opin Emerg Drugs. 2015; 20:161–4.
Article24. Urwyler A. Malignant hyperthermia: presymptomatic screening and treatment 2011. Eur J Anaesthesiol. 2011; 28:237–9.
Article25. Do Carmo PL, Zapata-Sudo G, Trachez MM, Das Graças Fernandes Sales M, Sudo RT. Toxicological evaluation of azumolene after repeated intraperitoneal administration in rats. Fundam Clin Pharmacol. 2010; 24:491–500.
Article26. Chen M, Wu Q, Jiang J, Jin X, Liu S, Wang M, et al. Preparation, characterization and in vivo evaluation of a formulation of dantrolene sodium with hydroxypropyl-β-cyclodextrin. J Pharm Biomed Anal. 2017; 135:153–9.
Article27. Sudo RT, Carmo PL, Trachez MM, Zapata-Sudo G. Effects of azumolene on normal and malignant hyperthermia-susceptible skeletal muscle. Basic Clin Pharmacol Toxicol. 2008; 102:308–16.
Article28. Ikemoto T, Hosoya T, Aoyama H, Kihara Y, Suzuki M, Endo M. Effects of dantrolene and its derivatives on Ca2+ release from the sarcoplasmic reticulum of mouse skeletal muscle fibres. Br J Pharmacol. 2001; 134:729–36.
Article29. Correia AC, Silva PC, Da Silva BA. Malignant hyperthermia: clinical and molecular aspects. Rev Bras Anestesiol. 2012; 62:820–37.
Article30. Van Petegem F. Ryanodine receptors: structure and function. J Biol Chem. 2012; 287:31624–32.
Article31. Muehlschlegel S, Sims JR. Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit Care. 2009; 10:103–15.
Article32. Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol. 2010; 2:a003996.
Article33. MacLennan DH, Duff C, Zorzato F, Fujii J, Phillips M, Korneluk RG, et al. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990; 343:559–61.
Article34. Xu SY, Hu FY, Ren LJ, Chen L, Zhou ZQ, Zhang XJ, et al. Dantrolene enhances the protective effect of hypothermia on cerebral cortex neurons. Neural Regen Res. 2015; 10:1279–85.
Article35. Fruen BR, Mickelson JR, Louis CF. Dantrolene inhibition of sarcoplasmic reticulum Ca2+ release by direct and specific actionat skeletal muscle ryanodine receptors. J Biol Chem. 1997; 272:26965–71.36. Mody I, MacDonald JF. NMDA receptor-dependent excitotoxicity: the role of intracellular Ca2+ release. Trends Pharmacol Sci. 1995; 16:356–9.37. Allen GC, Cattran CB, Peterson RG, Lalande M. Plasma levels of dantrolene following oral administration in malignant hyperthermia-susceptible patients. Anesthesiology. 1988; 69:900–4.
Article38. Flewellen EH, Nelson TE, Jones WP, Arens JF, Wagner DL. Dantrolene dose response in awake man: implications for management of malignant hyperthermia. Anesthesiology. 1983; 59:275–80.39. Dailymed. National Institutes of Health [Internet]. [updated 2006 Feb 6; cited 2023 Feb 20]. Available from https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=57b4eec1-49e1-432c-95ae-0fe36e32d64b.40. MHAUS. Malignant Hyperthermia Association of the United States [Internet]. 2018 [updated 2021 Feb; cited 2023 Feb 20]. Available from http://www.mhaus.org.41. Rüffert H, Bastian B, Bendixen D, Girard T, Heiderich S, Hellblom A, et al. Consensus guidelines on perioperative management of malignant hyperthermia suspected or susceptible patients from the European Malignant Hyperthermia Group. Br J Anaesth. 2021; 126:120–30.
Article42. Safety Committee of Japanese Society of Anesthesiologists. JSA guideline for the management of malignant hyperthermia crisis 2016. J Anesth. 2017; 31:307–17.43. Aderibigbe T, Lang BH, Rosenberg H, Chen Q, Li G. Cost-effectiveness analysis of stocking dantrolene in ambulatory surgery centers for the treatment of malignant hyperthermia. Anesthesiology. 2014; 120:1333–8.
Article44. Gong X. Malignant hyperthermia when dantrolene is not readily available. BMC Anesthesiol. 2021; 21:119.
Article45. Eagle pharmaceuticals. FDA approves eagle Pharmaceuticals’ Ryanodex for the treatment of malignant hyperthermia [Internet]. 2014 Jul 23 [2023 Feb 20]. Available from https://investor.eagleus.com/news-releases/news-release-details/fda-approves-eagle-pharmaceuticals-ryanodexr-treatment-malignant.46. Jeon J, Song S, Kim MC, Kim KM, Lee S. The effect of long-term oral dantrolene on the neuromuscular action of rocuronium: a case report. Korean J Anesthesiol. 2014; 66:153–6.
Article47. Driessen JJ, Wuis EW, Gielen MJ. Prolonged vecuronium neuromuscular blockade in a patient receiving orally administered dantrolene. Anesthesiology. 1985; 62:523–4.
Article48. Lynch C, Durbin CG, Fisher NA, Veselis RA, Althaus JS. Effects of dantrolene and verapamil on atrioventricular conduction and cardiovascular performance in dogs. Anesth Analg. 1986; 65:252–8.
Article49. Saltzman LS, Kates RA, Corke BC, Norfleet EA, Heath KR. Hyperkalemia and cardiovascular collapse after verapamil and dantrolene administration in swine. Anesth Analg. 1984; 63:473–8.
Article50. Rubin AS, Zablocki AD. Hyperkalemia, verapamil, and dantrolene. Anesthesiology. 1987; 66:246–9.
Article51. Yoganathan T, Casthely PA, Lamprou M. Dantrolene-induced hyperkalemia in a patient treated with diltiazem and metoprolol. J Cardiothorac Anesth. 1988; 2:363–4.
Article52. Freysz M, Timour Q, Bernaud C, Bertrix L, Faucon G. Cardiac implications of amlodipine-dantrolene combinations. Can J Anaesth. 1996; 43:50–5.
Article53. Tayeb OS. A serious interaction of dantrolene and theophylline. Vet Hum Toxicol. 1990; 32:442–3.54. Nogen AG. Medical treatment for spasticity in children with cerebral palsy. Childs Brain. 1976; 2:304–8.
Article55. Dantrolene Side Effects. Drugs.com [Internet]. [updated 2022 Oct 9; cited 2023 Feb 20]. Available from https://www.drugs.com/sfx/dantrolene-side-effects.html.56. Brandom BW, Larach MG, Chen MS, Young MC. Complications associated with the administration of dantrolene 1987 to 2006: a report from the North American Malignant Hyperthermia Registry of the Malignant Hyperthermia Association of the United States. Anesth Analg. 2011; 112:1115–23.57. Wedel DJ, Quinlan JG, Iaizzo PA. Clinical effects of intravenously administered dantrolene. Mayo Clin Proc. 1995; 70:241–6.
Article58. Durham JA, Gandolfi AJ, Bentley JB. Hepatotoxicological evaluation of dantrolene sodium. Drug Chem Toxicol. 1984; 7:23–40.
Article59. Ellis RH, Simpson P, Tatham P, Leighton M, Williams J. The cardiovascular effects of dantrolene sodium in dogs. Anaesthesia. 1975; 30:318–22.
Article60. EllIs KO, Butterfield JL, Wessels FL, Carpenter JF. A comparison of skeletal, cardiac, and smooth muscle actions of dantrolene sodium-a skeletal muscle relaxant. Arch Int Pharmacodyn Ther. 1976; 224:118–32.61. Lerman J, McLeod ME, Strong HA. Pharmacokinetics of intravenous dantrolene in children. Anesthesiology. 1989; 70:625–9.
Article62. Flewellen EH, Nelson TE. Dantrolene dose response in malignant hyperthermia-susceptible (MHS) swine: method to obtain prophylaxis and therapeusis. Anesthesiology. 1980; 52:303–8.63. Javed M, Bogdanov A. Oral dantrolene and severe respiratory failure in a patient with chronic spinal cord injury. Anaesthesia. 2010; 65:855–6.
Article64. Shime J, Gare D, Andrews J, Britt B. Dantrolene in pregnancy: lack of adverse effects on the fetus and newborn infant. Am J Obstet Gynecol. 1988; 159:831–4.
Article65. Peck J, Urits I, Crane J, McNally A, Noor N, Patel M, et al. Oral muscle relaxants for the treatment of chronic pain associated with cerebral palsy. Psychopharmacol Bull. 2020; 50(4 Suppl 1):142–62.66. Amano T, Fukami T, Ogiso T, Hirose D, Jones JP, Taniguchi T, et al. Identification of enzymes responsible for dantrolene metabolism in the human liver: A clue to uncover the cause of liver injury. Biochem Pharmacol. 2018; 151:69–78.
Article67. Chan CH. Dantrolene sodium and hepatic injury. Neurology. 1990; 40:1427–32.
Article68. Guglielminotti J, Rosenberg H, Li G. Prevalence of malignant hyperthermia diagnosis in obstetric patients in the United States, 2003 to 2014. BMC Anesthesiol. 2020; 20:19.
Article69. Conte-Camerino D, Lograno MD, Siro-Brigiani G, Megna G. Dantrolene sodium: stimulatory and depressant effects on the contractility of guinea pig uterus in vitro. Eur J Pharmacol. 1983; 92:291–4.70. Weingarten AE, Korsh JI, Neuman GG, Stern SB. Postpartum uterine atony after intravenous dantrolene. Anesth Analg. 1987; 66:269–70.
Article71. Dykes MH. Evaluation of a muscle relaxant: dantrolene sodium (Dantrium). JAMA. 1975; 231:862–4.
Article72. Shin YK, Kim YD, Collea JV, Belcher MD. Effect of dantrolene sodium on contractility of isolated human uterine muscle. Int J Obstet Anesth. 1995; 4:197–200.
Article73. Jr Craft JB, Goldberg NH, Lim M, Landsberger E, Mazel P, Abramson FP, et al. Cardiovascular effects and placental passage of dantrolene in the maternal-fetal sheep model. Anesthesiology. 1988; 68:68–72.
Article74. Fricker RM, Hoerauf KH, Drewe J, Kress HG. Secretion of dantrolene into breast milk after acute therapy of a suspected malignant hyperthermia crisis during cesarean section. Anesthesiology. 1998; 89:1023–5.
Article75. Verrotti A, Greco R, Spalice A, Chiarelli F, Iannetti P. Pharmacotherapy of spasticity in children with cerebral palsy. Pediatr Neurol. 2006; 34:1–6.
Article76. Mahoney JM, Bachtel MD. Pleural effusion associated with chronic dantrolene administration. Ann Pharmacother. 1994; 28:587–9.
Article77. See S, Ginzburg R. Skeletal muscle relaxants. Pharmacotherapy. 2008; 28:207–13.
Article78. Kheder A, Nair KP. Spasticity: pathophysiology, evaluation and management. Pract Neurol. 2012; 12:289–98.
Article79. Masi A, Narducci R, Mannaioni G. Harnessing ionic mechanisms to achieve disease modification in neurodegenerative disorders. Pharmacol Res. 2019; 147:104343.
Article80. Wang Y, Liang G, Liang S, Mund R, Shi Y, Wei H. Dantrolene ameliorates impaired neurogenesis and synaptogenesis in induced pluripotent stem cell lines derived from patients with Alzheimer’s disease. Anesthesiology. 2020; 132:1062–79.
Article81. Preckel B, Schlack W, Comfère T, Thämer V. Effect of dantrolene in an in vivo and in vitro model of myocardial reperfusion injury. Acta Anaesthesiol Scand. 2000; 44:194–201.
Article82. Pelletier MR, Wadia JS, Mills LR, Carlen PL. Seizure-induced cell death produced by repeated tetanic stimulation in vitro: possible role of endoplasmic reticulum calcium stores. J Neurophysiol. 1999; 81:3054–64.
Article83. Rutecki PA, Sayin U, Yang Y, Hadar E. Determinants of ictal epileptiform patterns in the hippocampal slice. Epilepsia. 2002; 43(Suppl 5):179–83.
Article84. Thorell WE, Leibrock LG, Agrawal SK. Role of RyRs and IP3 receptors after traumatic injury to spinal cord white matter. J Neurotrauma. 2002; 19:335–42.
Article85. Fernandez A, Schmidt JM, Claassen J, Pavlicova M, Huddleston D, Kreiter KT, et al. Fever after subarachnoid hemorrhage. Risk factors and impact on outcome. Neurology. 2007; 68:1013–9.86. Reith J, Jørgensen HS, Pedersen PM, Nakayama H, Raaschou HO, Jeppesen LL, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet. 1996; 347:422–5.
Article87. Lopez M, Sessler DI, Walter K, Emerick T, Ozaki M. Rate and gender dependence of the sweating, vasoconstriction, and shivering thresholds in humans. Anesthesiology. 1994; 80:780–8.
Article88. Siegmueller C, Narasimhaiah R. ‘Fatal 2,4-dinitrophenol poisoning... coming to a hospital near you’. Emerg Med J. 2010; 27:639–40.
Article89. Kopec KT, Kim T, Mowry J, Aks S, Kao L. Role of dantrolene in dinitrophenol (DNP) overdose: A continuing question? Am J Emerg Med. 2019; 37:1216. e1-2.
Article90. Hall AP, Henry JA. Acute toxic effects of ‘Ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth. 2006; 96:678–85.
Article91. Caroff SN, Watson CB, Rosenberg H. Drug-induced hyperthermic syndromes in psychiatry. Clin Psychopharmacol Neurosci. 2021; 19:1–11.
Article92. Musselman ME, Saely S. Diagnosis and treatment of drug-induced hyperthermia. Am J Health Syst Pharm. 2013; 70:34–42.
Article93. Ginz HF, Levano S, Girard T, Urwyler A, Hamel C. Dantrolene for severe rhabdomyolysis in Staphylococcus aureus toxic shock syndrome. Eur J Anaesthesiol. 2012; 29:161–2.
Article94. Chiba N, Matsuzaki M, Mawatari T, Mizuochi M, Sakurai A, Kinoshita K. Beneficial effects of dantrolene in the treatment of rhabdomyolysis as a potential late complication associated with COVID-19: a case report. Eur J Med Res. 2021; 26:18.
Article95. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020; 323:2052–9.
Article96. Grogan H, Hopkins PM. Heat stroke: implications for critical care and anaesthesia. Br J Anaesth. 2002; 88:700–7.
Article97. Köchling A, Wappler F, Winkler G, Schulte am Esch JS. Rhabdomyolysis following severe physical exercise in a patient with predisposition to malignant hyperthermia. Anaesth Intensive Care. 1998; 26:315–8.
Article98. Bouchama A. Heatstroke: a new look at an ancient disease. Intensive Care Med. 1995; 21:623–5.
Article99. Muldoon S, Deuster P, Voelkel M, Capacchione J, Bunger R. Exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia: is there a link? Curr Sports Med Rep. 2008; 7:74–80.
Article100. Tobin JR, Jason DR, Challa VR, Nelson TE, Sambuughin N. Malignant hyperthermia and apparent heat stroke. JAMA. 2001; 286:168–9.
Article101. Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995; 91:1512–9.
Article102. Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001; 103:196–200.
Article103. Penttinen K, Swan H, Vanninen S, Paavola J, Lahtinen AM, Kontula K, et al. Antiarrhythmic effects of dantrolene in patients with catecholaminergic polymorphic ventricular tachycardia and replication of the responses using iPSC models. PLoS One. 2015; 10:e0125366.
Article104. Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, et al. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol. 2009; 53:1993–2005.
Article105. Farkhondeh T, Roshanravan B, Shirazi FM, Mehrpour O. Can dantrolene be used in the treatment of cardioglycosides poisonings? Expert Opin Drug Metab Toxicol. 2021; 17:1–2.
Article106. Guengerich FP, MacDonald JS. Applying mechanisms of chemical toxicity to predict drug safety. Chem Res Toxicol. 2007; 20:344–69.
Article107. Dantrium® Intravenous. Procter & Gamble Pharmaceuticals, TM Owner. [Internet]. [cited 2023 Feb 20] Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/018264s025lbl.pdf.108. USFDA. Drug database; dantrolene sodium; Label–supplement 49 [Internet]. 2019 [cited 2023 Feb 20]. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205579s000lbl.pdf.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Dantrolene on the Interaction of Calcium and Erythrocyte Membrane
- Malignant hyperthermia and dantrolene sodium
- A suspected malignant hyperthermia managed without dantrolene sodium
- The Effects of Dantrolene Sodium in Prevention of Malignant Hyperthermia
- Suspicious Malignant Hyperthermia in Child during General Anesthesia: A case report