Endocrinol Metab.
2023 Oct;38(5):545-556. 10.3803/EnM.2023.1725.
Insulin Preferentially Regulates the Activity of Parasympathetic Preganglionic Neurons over Sympathetic Preganglionic Neurons
- Affiliations
-
- 1Department of Biological Sciences, Korea Advanced Institute of Science and Technology, Daejeon, Korea
- KMID: 2546985
- DOI: http://doi.org/10.3803/EnM.2023.1725
Abstract
- Background
Insulin is a peptide hormone that regulates post-prandial physiology, and it is well known that insulin controls homeostasis at least in part via the central nervous system. In particular, insulin alters the activity of neurons within the autonomic nervous system. However, currently available data are mostly from unidentified brainstem neurons of the dorsal motor nucleus of the vagus nerve (DMV).
Methods
In this study, we used several genetically engineered mouse models to label distinct populations of neurons within the brainstem and the spinal cord for whole-cell patch clamp recordings and to assess several in vivo metabolic functions.
Results
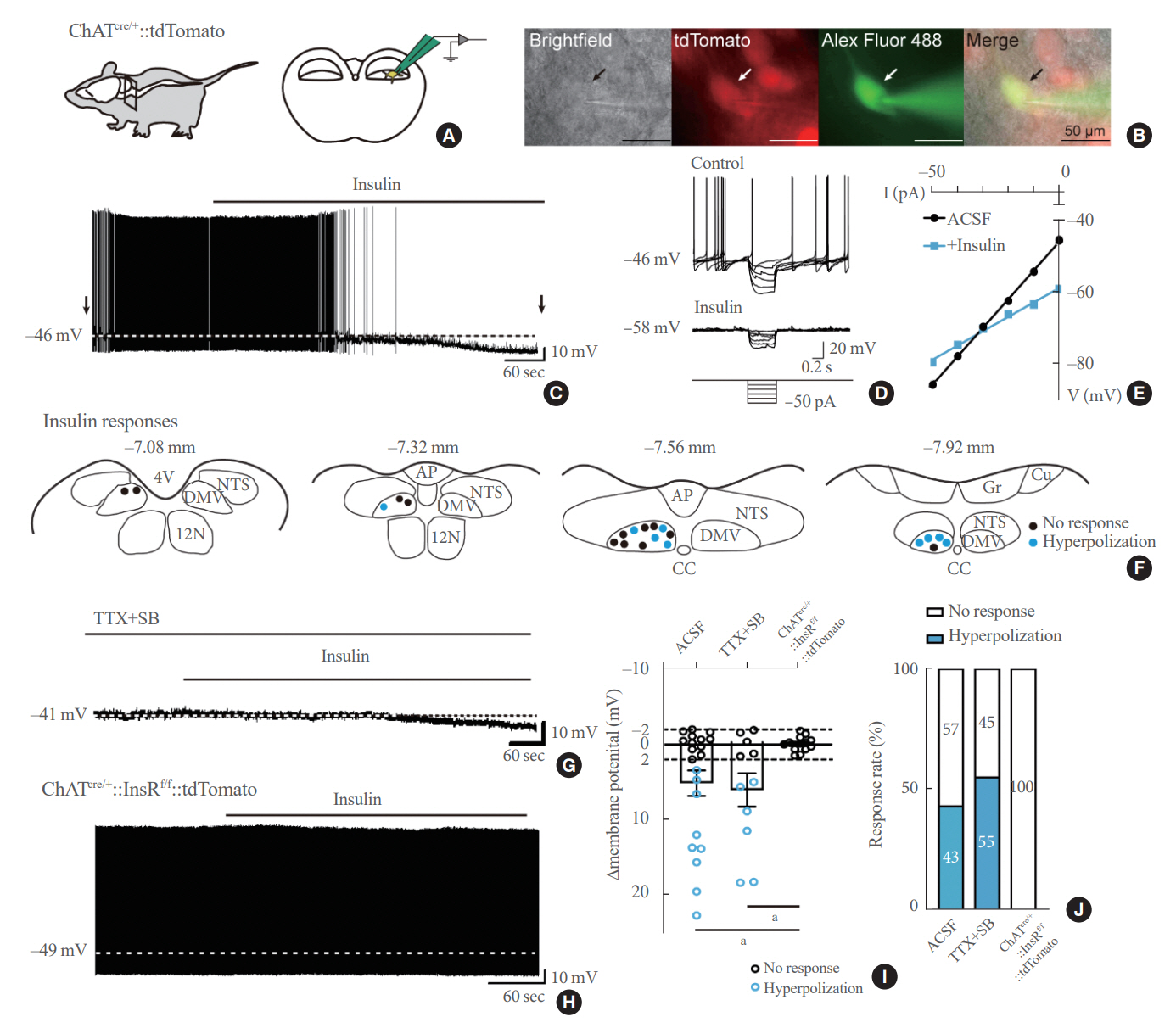
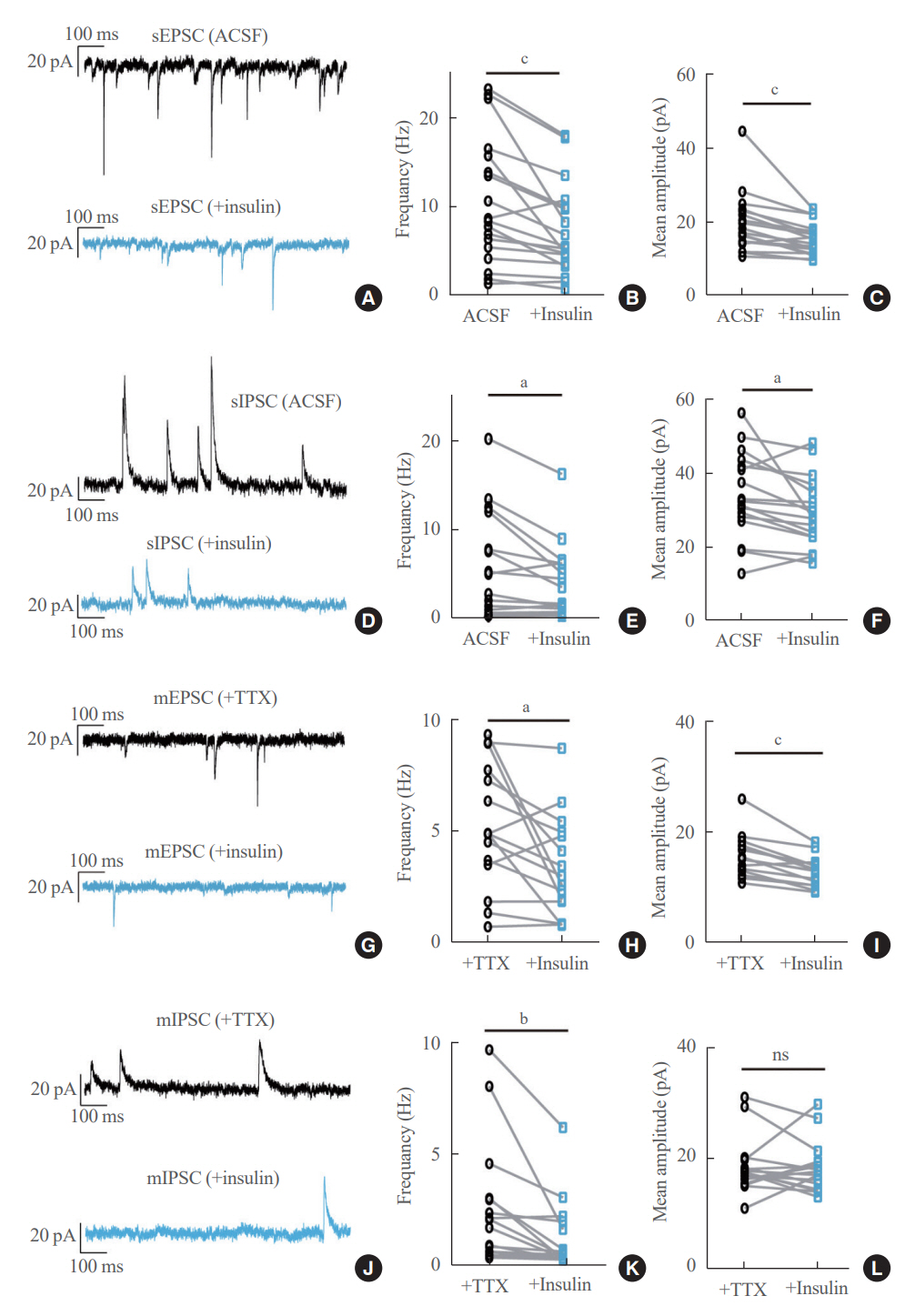
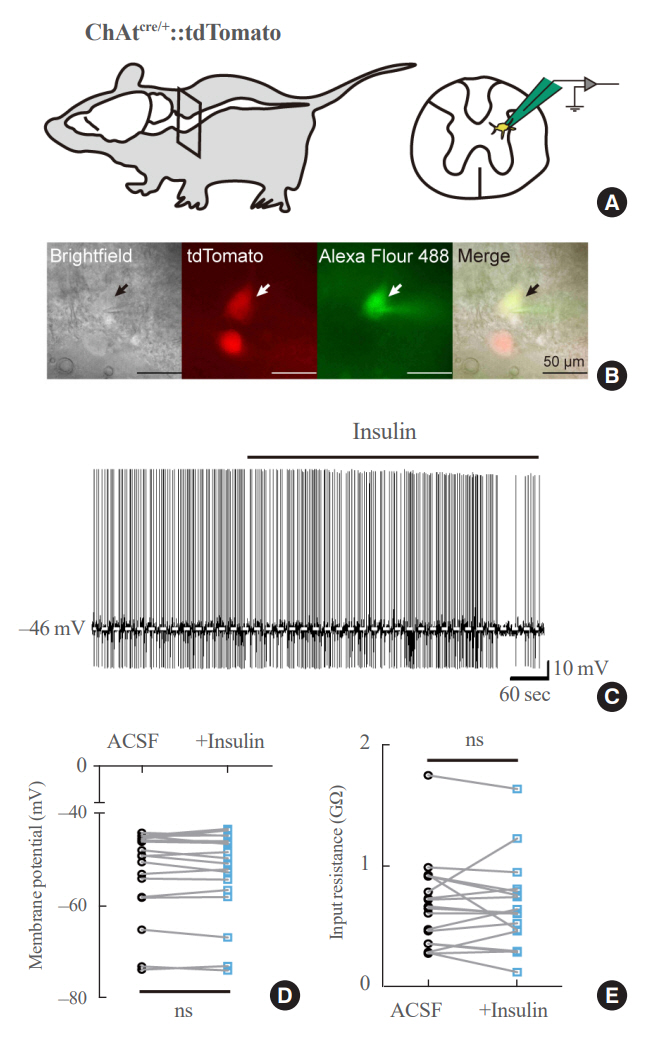
We first confirmed that insulin directly inhibited cholinergic (parasympathetic preganglionic) neurons in the DMV. We also found inhibitory effects of insulin on both the excitatory and inhibitory postsynaptic currents recorded in DMV cholinergic neurons. In addition, GABAergic neurons of the DMV and nucleus tractus solitarius were inhibited by insulin. However, insulin had no effects on the cholinergic sympathetic preganglionic neurons of the spinal cord. Finally, we obtained results suggesting that the insulininduced inhibition of parasympathetic preganglionic neurons may not play a critical role in the regulation of glucose homeostasis and gastrointestinal motility.
Conclusion
Our results demonstrate that insulin inhibits parasympathetic neuronal circuitry in the brainstem, while not affecting sympathetic neuronal activity in the spinal cord.
Keyword
Figure
Reference
-
1. Chen C, Cohrs CM, Stertmann J, Bozsak R, Speier S. Human beta cell mass and function in diabetes: recent advances in knowledge and technologies to understand disease pathogenesis. Mol Metab. 2017; 6:943–57.
Article2. Sonksen P, Sonksen J. Insulin: understanding its action in health and disease. Br J Anaesth. 2000; 85:69–79.
Article3. Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000; 289:2122–5.
Article4. Klockener T, Hess S, Belgardt BF, Paeger L, Verhagen LA, Husch A, et al. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat Neurosci. 2011; 14:911–8.
Article5. Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007; 5:438–49.
Article6. Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, et al. Direct insulin and leptin action on proopiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010; 11:286–97.
Article7. Filippi BM, Yang CS, Tang C, Lam TK. Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metab. 2012; 16:500–10.
Article8. Filippi BM, Bassiri A, Abraham MA, Duca FA, Yue JT, Lam TK. Insulin signals through the dorsal vagal complex to regulate energy balance. Diabetes. 2014; 63:892–9.
Article9. Blake CB, Smith BN. Insulin reduces excitation in gastricrelated neurons of the dorsal motor nucleus of the vagus. Am J Physiol Regul Integr Comp Physiol. 2012; 303:R807–14.
Article10. Grabauskas G, Moises HC. Gastrointestinal-projecting neurones in the dorsal motor nucleus of the vagus exhibit direct and viscerotopically organized sensitivity to orexin. J Physiol. 2003; 549(Pt 1):37–56.
Article11. Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S. Distribution and characterisation of glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab. 2015; 4:718–31.
Article12. Zheng Z, Lewis MW, Travagli RA. In vitro analysis of the effects of cholecystokinin on rat brain stem motoneurons. Am J Physiol Gastrointest Liver Physiol. 2005; 288:G1066–73.
Article13. Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, et al. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013; 152:612–9.
Article14. Ju SH, Yun H, Oh Y, Choi Y, Sohn JW. Melanocortin-4 receptors activate sympathetic preganglionic neurons and elevate blood pressure via TRPV1. Cell Rep. 2022; 41:111579.
Article15. Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, Greer T, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014; 306:E424–32.
Article16. Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011; 13:195–204.
Article17. Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010; 13:133–40.
Article18. Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998; 2:559–69.
Article19. Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, Takakura AC, et al. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol. 2007; 503:627–41.
Article20. Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res. 2004; 1017:208–17.
Article21. Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest. 2011; 121:2413–21.
Article22. Vong L, Ye C, Yang Z, Choi B, Chua S Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011; 71:142–54.
Article23. Hyun U, Sohn JW. Autonomic control of energy balance and glucose homeostasis. Exp Mol Med. 2022; 54:370–6.
Article24. Kwon E, Joung HY, Liu SM, Chua SC Jr, Schwartz GJ, Jo YH. Optogenetic stimulation of the liver-projecting melanocortinergic pathway promotes hepatic glucose production. Nat Commun. 2020; 11:6295.
Article25. Yang F, Liu Y, Chen S, Dai Z, Yang D, Gao D, et al. A GABAergic neural circuit in the ventromedial hypothalamus mediates chronic stress-induced bone loss. J Clin Invest. 2020; 130:6539–54.
Article26. Sohn JW, Oh Y, Kim KW, Lee S, Williams KW, Elmquist JK. Leptin and insulin engage specific PI3K subunits in hypothalamic SF1 neurons. Mol Metab. 2016; 5:669–79.
Article27. Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010; 30:2472–9.
Article28. Card JP, Enquist LW. Transneuronal circuit analysis with pseudorabies viruses. Curr Protoc Neurosci. 2014; 68:1.5.1–1.5.39.
Article29. Zhou SY, Lu YX, Yao H, Owyang C. Spatial organization of neurons in the dorsal motor nucleus of the vagus synapsing with intragastric cholinergic and nitric oxide/VIP neurons in the rat. Am J Physiol Gastrointest Liver Physiol. 2008; 294:G1201–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neurophysiology of Laryngopharyngeal Reflux and Brainstem Reflex
- The Frequency-Dependence of Pre- and Postganglionic Nerve Stimulation of Pig and Rat Bladder
- Sympathetic Excitation of Afferent Neurons within Dorsal Root Ganglia in a Rat Model of Sympathetically Medicated Pain
- Diagnostic Usefulness of Electrodiagnostic Study and Magnetic Resonance Imaging to Preganglionic Brachial Plexopathy
- Identification of ATP-sensitive K+ Conductances in Male Rat Major Pelvic Ganglion Neurons