J Korean Med Sci.
2023 Sep;38(37):e306. 10.3346/jkms.2023.38.e306.
Aortic Annulus Detection Based on Deep Learning for Transcatheter Aortic Valve Replacement Using Cardiac Computed Tomography
- Affiliations
-
- 1Department of Radiology, Korea University Anam Hospital, Seoul, Korea
- 2AI Center, Korea University Anam Hospital, Seoul, Korea
- 3Division of Cardiology, Department of Internal Medicine, Korea University Anam Hospital, Seoul, Korea
- 4National Heart and Lung Institute, Imperial College London, London, United Kingdom
- 5Cardiovascular Research Centre, Royal Brompton Hospital, London, United Kingdom
- 6Bioengineering Department and Imperial-X, Imperial College London, London, United Kingdom
- 7School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom
- KMID: 2546205
- DOI: http://doi.org/10.3346/jkms.2023.38.e306
Abstract
- Background
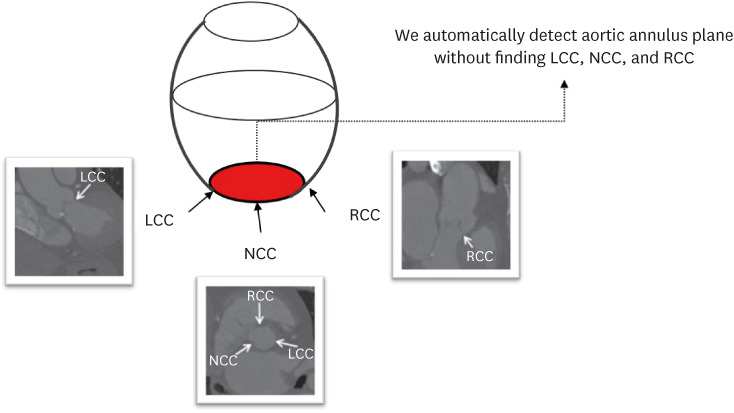
To propose a deep learning architecture for automatically detecting the complex structure of the aortic annulus plane using cardiac computed tomography (CT) for transcatheter aortic valve replacement (TAVR).
Methods
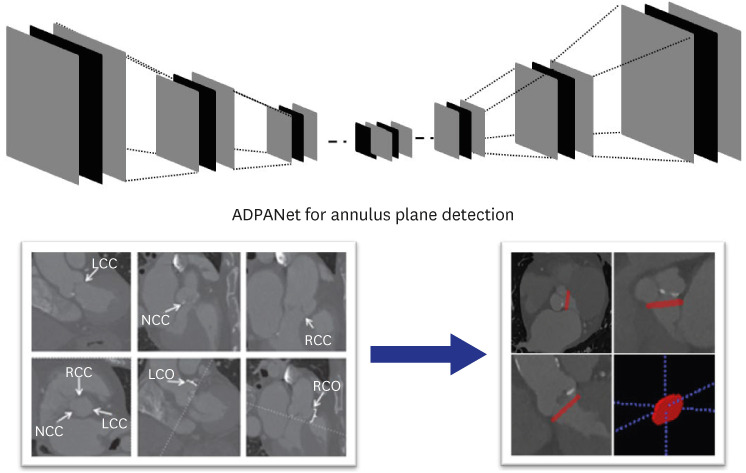
This study retrospectively reviewed consecutive patients who underwent TAVR between January 2017 and July 2020 at a tertiary medical center. Annulus Detection Permuted AdaIN network (ADPANet) based on a three-dimensional (3D) U-net architecture was developed to detect and localize the aortic annulus plane using cardiac CT. Patients (N = 72) who underwent TAVR between January 2017 and July 2020 at a tertiary medical center were enrolled. Ground truth using a limited dataset was delineated manually by three cardiac radiologists. Training, tuning, and testing sets (70:10:20) were used to build the deep learning model. The performance of ADPANet for detecting the aortic annulus plane was analyzed using the root mean square error (RMSE) and dice similarity coefficient (DSC).
Results
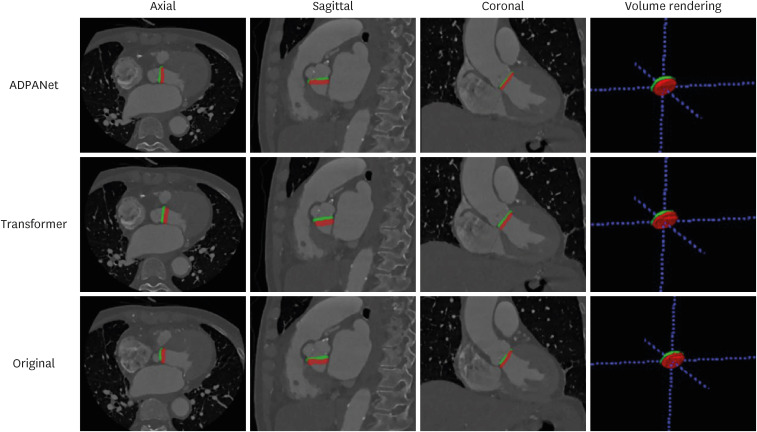
In this study, the total dataset consisted of 72 selected scans from patients who underwent TAVR. The RMSE and DSC values for the aortic annulus plane using ADPANet were 55.078 ± 35.794 and 0.496 ± 0.217, respectively.
Conclusion
Our deep learning framework was feasible to detect the 3D complex structure of the aortic annulus plane using cardiac CT for TAVR. The performance of our algorithms was higher than other convolutional neural networks.
Figure
Reference
-
1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006; 368(9540):1005–1011. PMID: 16980116.2. In : Palmer RI, Xie X, Tam GKL, editors. Automatic aortic root segmentation with shape constraints and mesh regularisation. Proceedings of the British Machine Vision Conference 2015; 2015 Sep 7-10; Swansea, UK. Durham: BMVA Press;2015.3. Liang L, Kong F, Martin C, Pham T, Wang Q, Duncan J, et al. Machine learning-based 3-D geometry reconstruction and modeling of aortic valve deformation using 3-D computed tomography images. Int J Numer Method Biomed Eng. 2017; 33(5):e2827.
Article4. Leng S, Zhang S, Jiang M, Zhao X, Wu R, Allen J, et al. Imaging 4D morphology and dynamics of mitral annulus in humans using cardiac cine MR feature tracking. Sci Rep. 2018; 8(1):81. PMID: 29311562.5. Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S. Cardiovascular magnetic resonance myocardial feature tracking: concepts and clinical applications. Circ Cardiovasc Imaging. 2016; 9(4):e004077. PMID: 27009468.6. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015; 521(7553):436–444. PMID: 26017442.
Article7. Schmidhuber J. Deep learning in neural networks: an overview. Neural Netw. 2015; 61:85–117. PMID: 25462637.
Article8. Kerfoot E, King CE, Ismail T, Nordsletten D, Miller R. Estimation of Cardiac Valve Annuli Motion With Deep Learning. Statistical Atlases and Computational Models of the Heart. M&Ms and EMIDEC Challenges. Updated 2021. Accessed January 29, 2021. http://www.who.int/whosis/en/menu.cfm .9. In : Chen H, Qi X, Cheng JZ, Heng PA, editors. Deep contextual networks for neuronal structure segmentation. Proceedings of the AAAI Conference on Artificial Intelligence; 2016 Feb 12-17; Phoenix, AZ, USA. Washington, D.C: AAAI Press;2016.10. Rao M, Stough J, Chi YY, Muller K, Tracton G, Pizer SM, et al. Comparison of human and automatic segmentations of kidneys from CT images. Int J Radiat Oncol Biol Phys. 2005; 61(3):954–960. PMID: 15708280.
Article11. In : Ronneberger O, Fischer P, Brox T, editors. U-net: convolutional networks for biomedical image segmentation. Medical Image Computing and Computer-Assisted Intervention -- MICCAI 2015: 18th International Conference; 2015 Oct 5-9; Munich, Germany. Cham, Switzerland: Springer International Publishing;2015.12. In : Noh H, Hong S, Han B, editors. Learning deconvolution network for semantic segmentation. Proceedings of the IEEE International Conference on Computer Vision; 2015 Dec 7-13; Santiago, Chile. Manhattan: Institute of Electrical and Electronics Engineers (IEEE);2015. p. 1520–1528.13. Shelhamer E, Long J, Darrell T. Fully convolutional networks for semantic segmentation. IEEE Trans Pattern Anal Mach Intell. 2017; 39(4):640–651. PMID: 27244717.
Article14. Chen LC, Papandreou G, Kokkinos I, Murphy K, Yuille AL. Deeplab: Semantic image segmentation with deep convolutional nets, atrous convolution, and fully connected CRFs. IEEE Trans Pattern Anal Mach Intell. 2018; 40(4):834–848. PMID: 28463186.
Article15. In : Çiçek Ö, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger O, editors. 3D U-net: learning dense volumetric segmentation from sparse annotation. Medical Image Computing and Computer-Assisted Intervention -- MICCAI 2016: 19th International Conference International Conference; 2016 Oct 17-21; Athens, Greece. Cham, Switzerland: Springer International Publishing;2016. p. 424–432.16. Isensee F, Petersen J, Kohl SA, Jäger PF, Maier-Hein KH. nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat Methods. 2021; 18(2):203–211. PMID: 33288961.
Article17. Nuriel O, Benaim S, Wolf L. Permuted AdaIN: Reducing the Bias Towards Global Statistics in Image Classification, IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR). 2021.18. Christ PF, Elshaer ME, Ettlinger F, Tatavarty S, Bickel M, Bilic P, et al. Automatic liver and lesion segmentation in CT using cascaded fully convolutional neural networks and 3D conditional random fields. In : Medical Image Computing and Computer-Assisted Intervention -- MICCAI 2016: 19th International Conference International Conference; 2016 Oct 17-21; Athens, Greece. Cham, Switzerland: Springer International Publishing;2016. p. 415–423.19. Cui S, Mao L, Jiang J, Liu C, Xiong S. Automatic semantic segmentation of brain gliomas from MRI images using a deep cascaded neural network. J Healthc Eng. 2018; 2018:4940593. PMID: 29755716.
Article20. He Y, Carass A, Yun Y, Zhao C, Jedynak BM, Solomon SD, et al. Towards topological correct segmentation of macular OCT from cascaded FCNs. Fetal Infant Ophthalmic Med Image Anal. 2017; 10554:202–209.21. Roth HR, Oda H, Zhou X, Shimizu N, Yang Y, Hayashi Y, et al. An application of cascaded 3D fully convolutional networks for medical image segmentation. Comput Med Imaging Graph. 2018; 66:90–99. PMID: 29573583.22. In : Tang M, Zhang Z, Cobzas D, Jagersand M, Jaremko JL, editors. Segmentation-by-detection: a cascade network for volumetric medical image segmentation. 2018 IEEE 15th International Symposium on Biomedical Imaging; 2018 Apr 4-7; Washington, D.C., USA. Manhattan: IEEE Publications;2018. p. 1356–1359.23. Wang G, Li W, Zuluaga MA, Pratt R, Patel PA, Aertsen M, et al. Interactive medical image segmentation using deep learning with image-specific fine tuning. IEEE Trans Med Imaging. 2018; 37(7):1562–1573. PMID: 29969407.
Article24. Penso M, Moccia S, Scafuri S, Muscogiuri G, Pontone G, Pepi M, et al. Automated left and right ventricular chamber segmentation in cardiac magnetic resonance images using dense fully convolutional neural network. Comput Methods Programs Biomed. 2021; 204:106059. PMID: 33812305.
Article25. Zhang X, Noga M, Martin DG, Punithakumar K. Fully automated left atrium segmentation from anatomical cine long-axis MRI sequences using deep convolutional neural network with unscented Kalman filter. Med Image Anal. 2021; 68:101916. PMID: 33285484.
Article26. Astudillo P, Mortier P, Bosmans J, De Backer O, de Jaegere P, Iannaccone F, et al. Automatic detection of the aortic annular plane and coronary ostia from multidetector computed tomography. J Interv Cardiol. 2020; 2020:9843275. PMID: 32549802.
Article27. In : Xie Y, Zhang J, Shen C, Xia Y, editors. CoTr: efficiently bridging CNN and transformer for 3D medical image segmentation. Medical Image Computing and Computer-Assisted Intervention -- MICCAI 2021: 24th International Conference; 2021 Sep 27-Oct 1; Strasbourg, France. Cham, Switzerland: Springer International Publishing;2021. p. 171–180.28. Auricchio F, Conti M, Morganti S, Reali A. Simulation of transcatheter aortic valve implantation: a patient-specific finite element approach. Comput Methods Biomech Biomed Engin. 2014; 17(12):1347–1357. PMID: 23402555.
Article29. Capelli C, Bosi GM, Cerri E, Nordmeyer J, Odenwald T, Bonhoeffer P, et al. Patient-specific simulations of transcatheter aortic valve stent implantation. Med Biol Eng Comput. 2012; 50(2):183–192. PMID: 22286953.
Article30. Dwyer HA, Matthews PB, Azadani A, Ge L, Guy TS, Tseng EE. Migration forces of transcatheter aortic valves in patients with noncalcific aortic insufficiency. J Thorac Cardiovasc Surg. 2009; 138(5):1227–1233. PMID: 19748632.
Article31. Morganti S, Conti M, Aiello M, Valentini A, Mazzola A, Reali A, et al. Simulation of transcatheter aortic valve implantation through patient-specific finite element analysis: two clinical cases. J Biomech. 2014; 47(11):2547–2555. PMID: 24998989.
Article32. Wang Q, Kodali S, Primiano C, Sun W. Simulations of transcatheter aortic valve implantation: implications for aortic root rupture. Biomech Model Mechanobiol. 2015; 14(1):29–38. PMID: 24736808.
Article33. Wang Q, Sirois E, Sun W. Patient-specific modeling of biomechanical interaction in transcatheter aortic valve deployment. J Biomech. 2012; 45(11):1965–1971. PMID: 22698832.34. Morgan TG, Bostrom MP, van der Meulen MC. Tissue-level remodeling simulations of cancellous bone capture effects of in vivo loading in a rabbit model. J Biomech. 2015; 48(5):875–882. PMID: 25579991.35. de Jaegere P, De Santis G, Rodriguez-Olivares R, Bosmans J, Bruining N, Dezutter T, et al. Patient-specific computer modeling to predict aortic regurgitation after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2016; 9(5):508–512. PMID: 26965945.
Article36. Huang X, Dione DP, Compas CB, Papademetris X, Lin BA, Bregasi A, et al. Contour tracking in echocardiographic sequences via sparse representation and dictionary learning. Med Image Anal. 2014; 18(2):253–271. PMID: 24292554.
Article37. Zhu Y, Papademetris X, Sinusas AJ, Duncan JS. Segmentation of the left ventricle from cardiac MR images using a subject-specific dynamical model. IEEE Trans Med Imaging. 2010; 29(3):669–687. PMID: 19789107.
Article38. Ecabert O, Peters J, Weese J. Modeling Shape Variability for Full Heart Segmentation in Cardiac Computed-Tomography Images. Bellingham, WA, USA: SPIE;2006.39. Zheng Y, Barbu A, Georgescu B, Scheuering M, Comaniciu D. Four-chamber heart modeling and automatic segmentation for 3-D cardiac CT volumes using marginal space learning and steerable features. IEEE Trans Med Imaging. 2008; 27(11):1668–1681. PMID: 18955181.
Article40. Lorenz C, von Berg J. A comprehensive shape model of the heart. Med Image Anal. 2006; 10(4):657–670. PMID: 16709463.41. In : Lin N, Papademetris X, Sinusas AJ, Duncan JS, editors. Analysis of left ventricular motion using a general robust point matching algorithm. Medical Image Computing and Computer-Assisted Intervention -- MICCAI 2003: 6th International Conference; 2003 Nov 15-18; Montréal, Canada. Berlin: Springer International Publishing;2003. p. 556–563.42. Schneider RJ, Perrin DP, Vasilyev NV, Marx GR, del Nido PJ, Howe RD. Mitral annulus segmentation from four-dimensional ultrasound using a valve state predictor and constrained optical flow. Med Image Anal. 2012; 16(2):497–504. PMID: 22200622.43. Zheng Y, John M, Liao R, Nöttling A, Boese J, Kempfert J, et al. Automatic aorta segmentation and valve landmark detection in C-arm CT for transcatheter aortic valve implantation. IEEE Trans Med Imaging. 2012; 31(12):2307–2321. PMID: 22955891.44. Pouch AM, Wang H, Takabe M, Jackson BM, Sehgal CM, Gorman JH 3rd, et al. Automated segmentation and geometrical modeling of the tricuspid aortic valve in 3D echocardiographic images. Med Image Comput Comput Assist Interv. 2013; 16(Pt 1):485–492.45. Ionasec RI, Voigt I, Georgescu B, Wang Y, Houle H, Vega-Higuera F, et al. Patient-specific modeling and quantification of the aortic and mitral valves from 4-D cardiac CT and TEE. IEEE Trans Med Imaging. 2010; 29(9):1636–1651. PMID: 20442044.
Article46. Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol. 1987; 59(9):998–999. PMID: 3565291.47. Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993; 21(5):1220–1225. PMID: 8459080.
Article48. Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997; 29(3):630–634. PMID: 9060903.49. Agmon Y, Khandheria BK, Meissner I, Sicks JR, O’Fallon WM, Wiebers DO, et al. Aortic valve sclerosis and aortic atherosclerosis: different manifestations of the same disease? Insights from a population-based study. J Am Coll Cardiol. 2001; 38(3):827–834. PMID: 11527641.
Article50. Ferreira-González I, Pinar-Sopena J, Ribera A, Marsal JR, Cascant P, González-Alujas T, et al. Prevalence of calcific aortic valve disease in the elderly and associated risk factors: a population-based study in a Mediterranean area. Eur J Prev Cardiol. 2013; 20(6):1022–1030. PMID: 22679252.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expanding transcatheter aortic valve replacement into uncharted indications
- Recent updates in transcatheter aortic valve implantation
- Comparison of Transcatheter Aortic Valve Replacement between SelfExpanding versus Balloon-Expandable Valves in Patients with Small Aortic Annulus
- Aortic Valve Sparing Operations: A Review
- Successful Transcatheter Aortic Valve Replacement for Severe Aortic Regurgitation after CARVAR Operation