J Cerebrovasc Endovasc Neurosurg.
2023 Sep;25(3):275-287. 10.7461/jcen.2023.E2022.10.010.
Analysis of reported adverse events of pipeline stents for intracranial aneurysms using the FDA MAUDE database
- Affiliations
-
- 1Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, USA
- KMID: 2546164
- DOI: http://doi.org/10.7461/jcen.2023.E2022.10.010
Abstract
Objective
Flow diverting stents (FDS) are a validated device in the treatment of intracranial aneurysms, allowing for minimally invasive intervention. However, after its approval for use in the United States in 2011, post-market surveillance of adverse events is limited. This study aims to address this critical knowledge gap by analyzing the FDA Manufacturer and User Facility Device Experience (MAUDE) database for patient and device related (PR and DR) reports of adverse events and malfunctions.
Methods
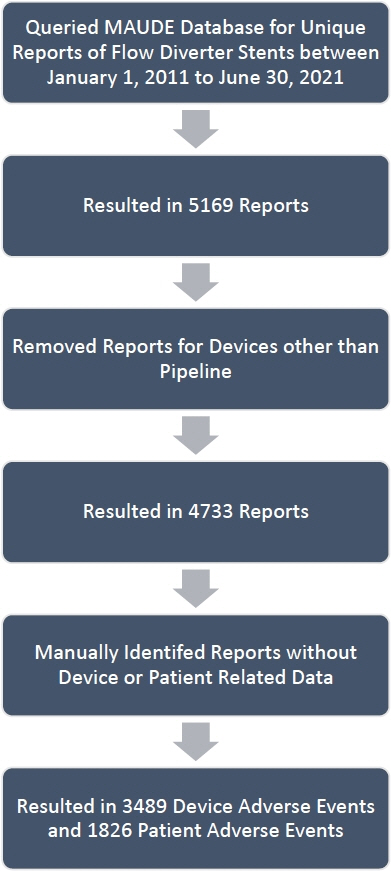
Using post-market surveillance data from the MAUDE database, PR and DR reports from January 2012-December 2021 were extracted, compiled, and analyzed with R-Studio version 2021.09.2. PR and DR reports with insufficient information were excluded. Raw information was organized, and further author generated classifications were created for both PR and DR reports.
Results
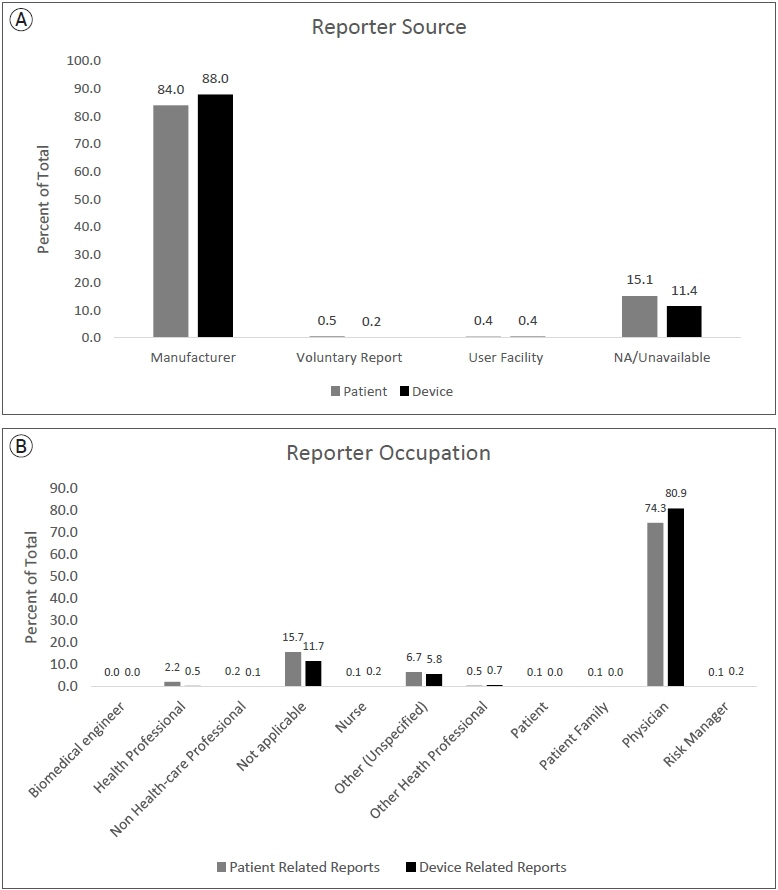
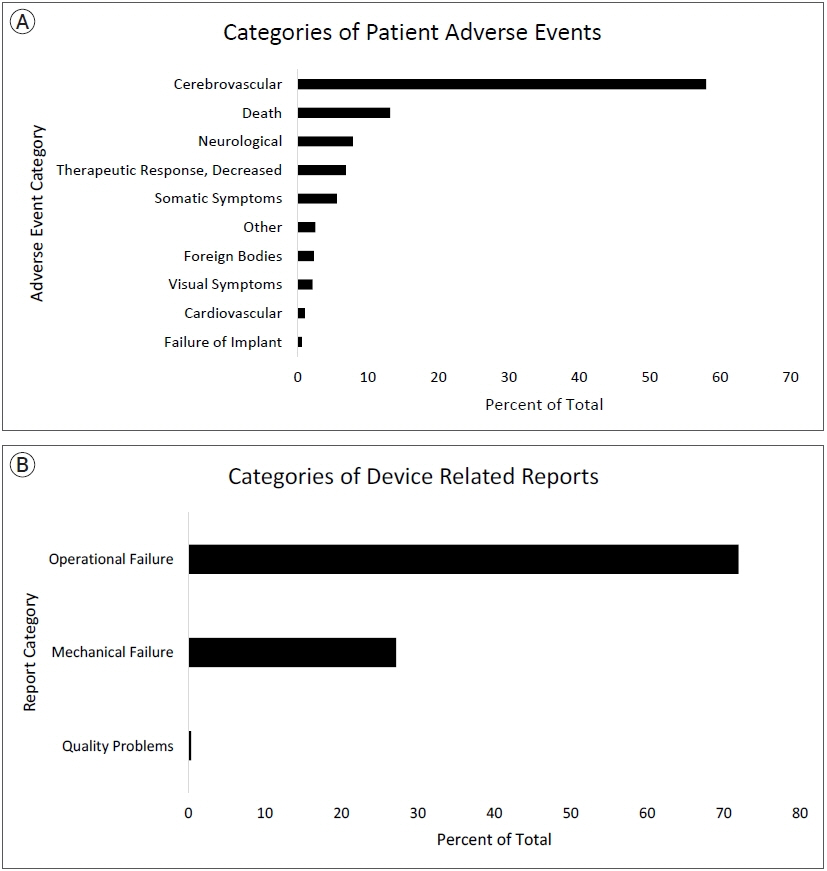
A total of 2203 PR and 4017 DR events were recorded. The most frequently reported PR adverse event categories were cerebrovascular (60%), death (11%), and neurological (8%). The most frequent PR adverse event reports were death (11%), thrombosis/thrombus (9%) cerebral infarction (8%), decreased therapeutic response (7%), stroke/cerebrovascular accident (6%), intracranial hemorrhage (5%), aneurysm (4%), occlusion (4%), headache (4%), neurological deficit/dysfunction (3%). The most frequent DR reports were activation/positioning/separation problems (52%), break (9%), device operates differently than expected (4%), difficult to open or close (4%), material deformation (3%), migration or expulsion of device (3%), detachment of device or device component (2%).
Conclusions
Post-market surveillance is important to guide patient counselling and identify adverse events and device problems that were not identified in initial trials. We present frequent reports of several types of cerebrovascular and neurological adverse events as well as the most common device shortcomings that should be explored by manufacturers and future studies. Although inherent limitations to the MAUDE database are present, our results highlight important PR and DR complications that can help optimize patient counseling and device development.
Keyword
Figure
Reference
-
1. Akgul E, Onan HB, Bilgin SS, Tahta A, Khanmammadov E, Gungoren FZ, et al. Flow diverter stents in the treatment of cerebral aneurysms less than 5 mm. Turk Neurosurg. 2021; 31(1):31–7.2. Badnjević A, Pokvić LG, Deumić A, Bećirović LS. Postmarket surveillance of medical devices: a review. Technol Health Care. 2022; 30(6):1315–29.3. Brewer T, Colditz GA. Postmarketing surveillance and adverse drug reactions: current perspectives and future needs. JAMA. 1999; Mar. 281(9):824–9.4. Chalouhi N, Jabbour P, Singhal S, Drueding R, Starke RM, Dalyai RT, et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke. 2013; May. 44(5):1348–53.5. Charbonnier G, Desilles JP, Escalard S, Maier B, Ciccio G, Smajda S, et al. Timing and spectrum of neurological complications after flow diverter implantation for intracranial aneurysms. Front Neurol. 2021; Apr. 12:590383.6. Charbonnier G, Desilles JP, Escalard S, Maier B, Ciccio G, Smajda S, et al. Timing and spectrum of neurological complications after flow diverter implantation for intracranial aneurysms. Front Neurol. 2021; Apr. 12:590383.7. Chiu AH, Nadarajah M, Wenderoth JD. Cost analysis of intracranial aneurysmal repair by endovascular coiling versus flow diversion: at what size should we use which method? JMed Imaging Radiat Oncol. 2013; Aug. 57(4):423–6.8. Cottier JP, Pasco A, Gallas S, Gabrillargues J, Cognard C, Drouineau J, et al. Utility of balloon-assisted Guglielmi detachable coiling in the treatment of 49 cerebral aneurysms: a retrospective, multicenter study. AJNR Am J Neuroradiol. 2001; 22(2):345–51.9. Dowlati E, Pasko KBD, Liu J, Miller CA, Felbaum DR, Sur S, et al. Treatment of in-stent stenosis following flow diversion of intracranial aneurysms with cilostazol and clopidogrel. Neurointervention. 2021; Nov. 16(3):285–92.10. Ensign LG, Cohen KB. A primer to the structure, content and linkage of the FDA’s manufacturer and user facility device experience (MAUDE) files. eGEMs. 2017; Jun. 5(1):12.11. Ev3 Inc. Instructions for use (IFU) pipeline embolization device. 2011. Apr. p. DRAFT IFU-0010.12. FDA. MAUDE - manufacturer and user facility device experience. Accessed April 30, 2022. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm.13. Fraser AG. Postmarket surveillance of high-risk medical devices needs transparent, comprehensive and independent registries. BMJ Surg Interv Health Technol. 2020; Oct. 2(1):e000065.14. Giles TX, Bennet J, Stone CE, Gendreau JL, Abraham M, Mammis A. Characterizing complications of intracranial responsive neurostimulation devices for epilepsy through a retrospective analysis of the federal MAUDE database. Neuromodulation. 2022; Feb. 25(2):263–70.15. Gurtcheff SE. Introduction to the MAUDE database. Clin Obstet Gynecol. 2008; Mar. 51(1):120–3.16. Hauser RG, Kallinen LM, Almquist AK, Gornick CC, Katsiyiannis WT. Early failure of a small-diameter high-voltage implantable cardioverter-defibrillator lead. Heart Rhythm. 2007; Jul. 4(7):892–6.17. Iwaishi C, Iwasaki K. A comprehensive analysis of postmarket surveillance study orders: device characteristics, study statuses, outcomes, and potential contributions. Ther Innov Regul Sci. 2020; Jul. 54(4):953–63.18. Kavanagh KT, Brown RE Jr, Kraman SS, Calderon LE, Kavanagh SP. Reporter’s occupation and source of adverse device event reports contained in the FDA’s MAUDE database. Patient Relat Outcome Meas. 2019; Jul. 10:205–8.19. Leung GKK, Tsang ACO, Lui WM. Pipeline embolization device for intracranial aneurysm: a systematic review. Clin Neuroradiol. 2012; Dec. 22(4):295–303.20. Normand SLT, Hatfield L, Drozda J, Resnic FS. Postmarket surveillance for medical devices: America’s new strategy. BMJ. 2012; Oct. 345:e6864.21. Pane J, Francisca RDC, Verhamme KMC, Orozco M, Viroux H, Rebollo I, et al. EU postmarket surveillance plans for medical devices. Pharmacoepidemiol Drug Saf. 2019; Sep. 28(9):1155–65.22. Phillips TJ, Wenderoth JD, Phatouros CC, Rice H, Singh TP, Devilliers L, et al. Safety of the pipeline embolization device in treatment of posterior circulation aneurysms. AJNR Am J Neuroradiol. 2012; Aug. 33(7):1225–31.23. Pressman E, de La Garza CA, Chin F, Fishbein J, Waqas M, Siddiqui A, et al. Nuisance bleeding complications in patients with cerebral aneurysm treated with Pipeline embolization device. J Neurointerv Surg. 2021; Mar. 13(3):247–50.24. Shin DS, Carroll CP, Elghareeb M, Hoh BL, Kim BT. The evolution of flow-diverting stents for cerebral aneurysms; historical review, modern application, complications, and future direction. J Korean Neurosurg Soc. 2020; Mar. 63(2):137–52.25. Trivelato FP, Wajnberg E, Rezende MTS, Ulhôa AC, Piske RL, Abud TG, et al. Safety and effectiveness of the pipeline flex embolization device with shield technology for the treatment of intracranial aneurysms: midterm results from a multicenter study. Neurosurgery. 2020; Jul. 87(1):104–11.26. Wali AR, Park CC, Santiago-Dieppa DR, Vaida F, Murphy JD, Khalessi AA. Pipeline embolization device versus coiling for the treatment of large and giant unruptured intracranial aneurysms: a cost-effectiveness analysis. Neurosurg Focus. 2017; Jun. 42(6):e6.27. Yu SCH, Kwok CK, Cheng PW, Chan KY, Lau SS, Lui WM, et al. Intracranial aneurysms: midterm outcome of pipeline embolization device - a prospective study in 143 patients with 178 aneurysms. Radiology. 2012; Dec. 265(3):893–901.28. Zhou G, Su M, Yin YL, Li MH. Complications associated with the use of flow-diverting devices for cerebral aneurysms: a systematic review and meta-analysis. Neurosurg Focus. 2017; Jun. 42(6):e17.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adverse Events Associated With Synthetic Male Slings: An Analysis of the Food and Drug Administration Manufacturer and User Facility Device Experience Database
- Real world adverse events of interspinous spacers using Manufacturer and User Facility Device Experience data
- Analysis of Important Medical Adverse Events and Signals Related with Cyclosporine and Tacrolimus Using the FDA Adverse Event Reporting System (FAERS) Database
- An evaluation of the Manufacturer And User Facility Device Experience database that inspired the United States Food and Drug Administration's Reclassification of transvaginal mesh
- The Evolution of Flow-Diverting Stents for Cerebral Aneurysms; Historical Review, Modern Application, Complications, and Future Direction