Diabetes Metab J.
2023 Sep;47(5):612-629. 10.4093/dmj.2023.0067.
Dyslipidemia in Patients with Chronic Kidney Disease: An Updated Overview
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea
- KMID: 2546120
- DOI: http://doi.org/10.4093/dmj.2023.0067
Abstract
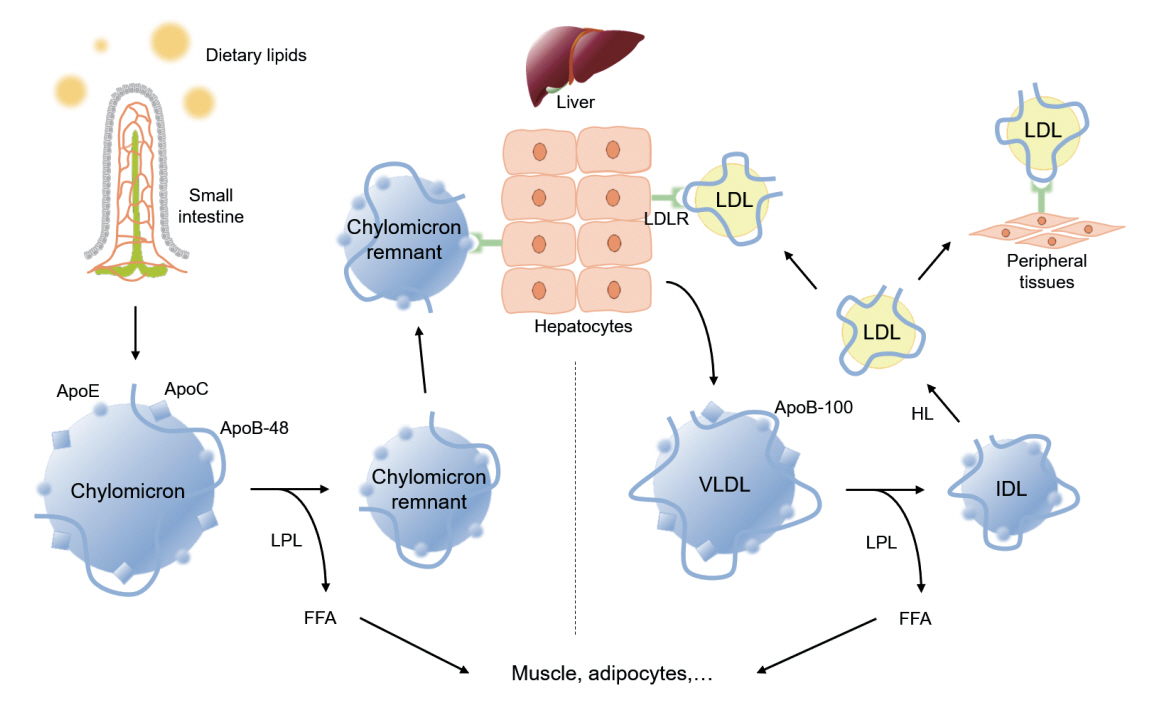
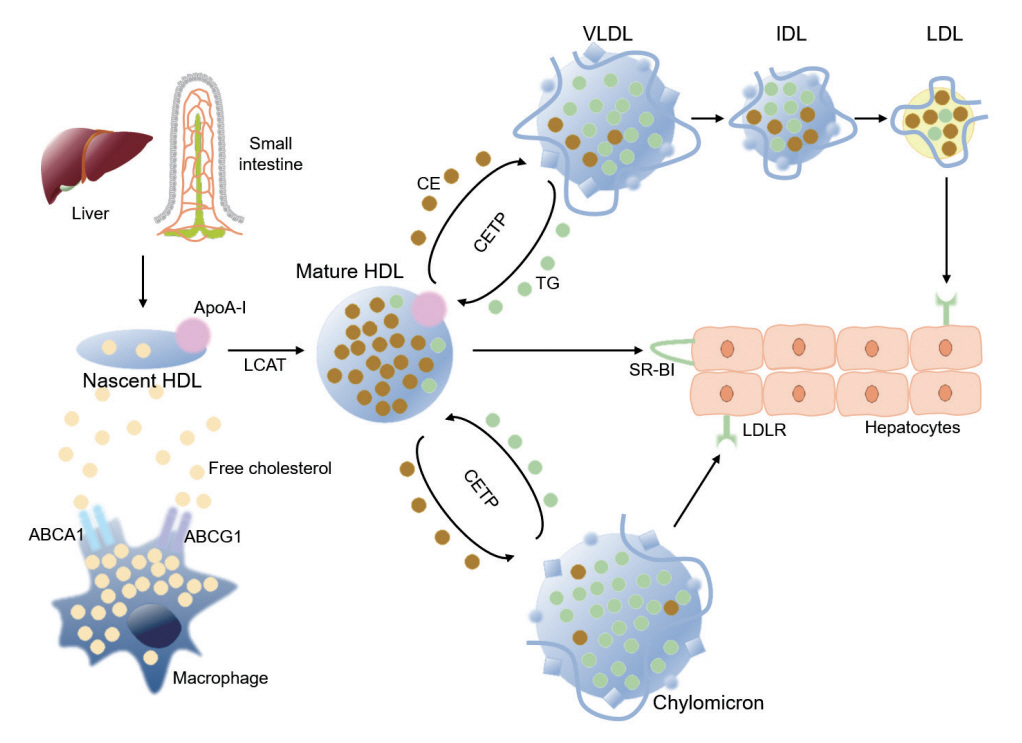
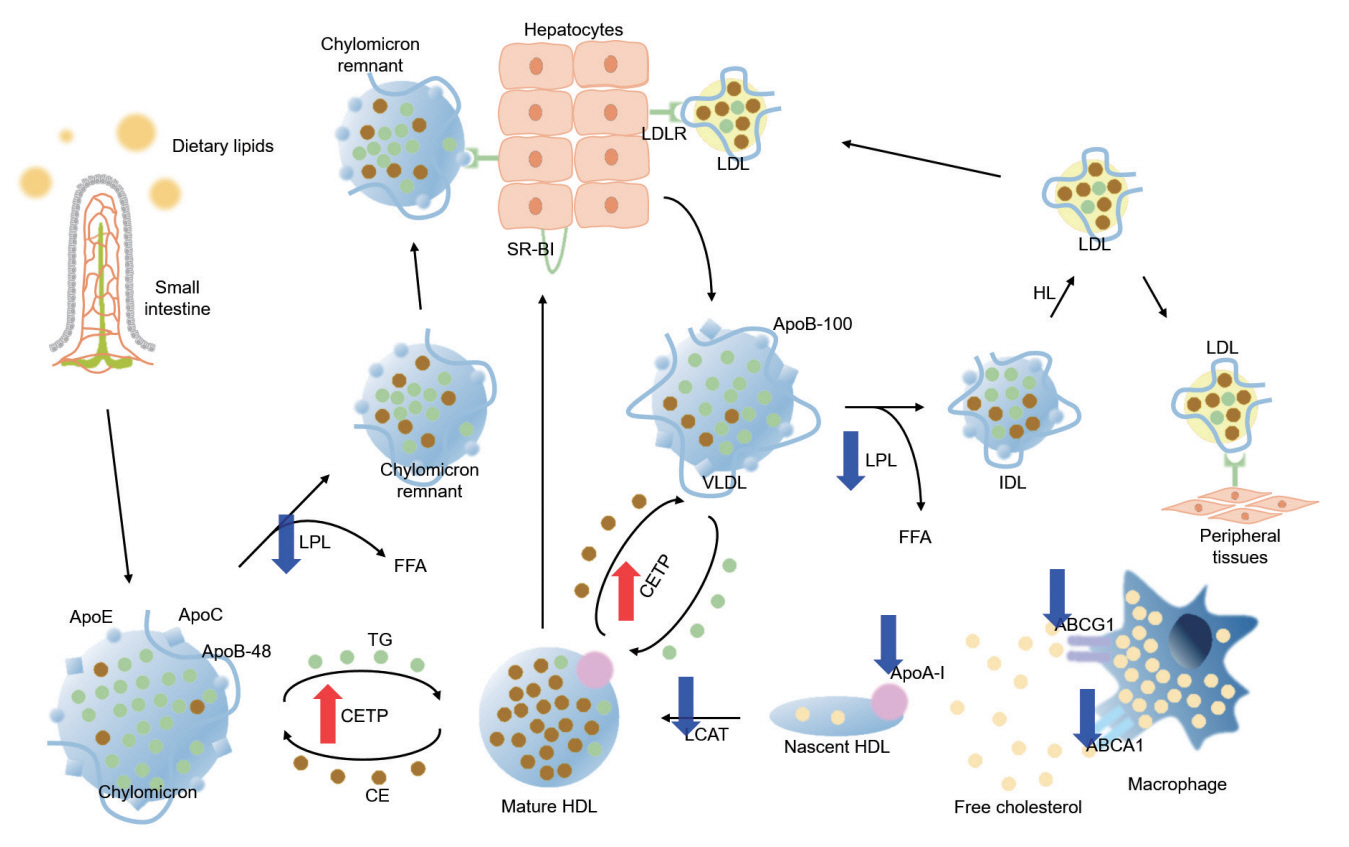
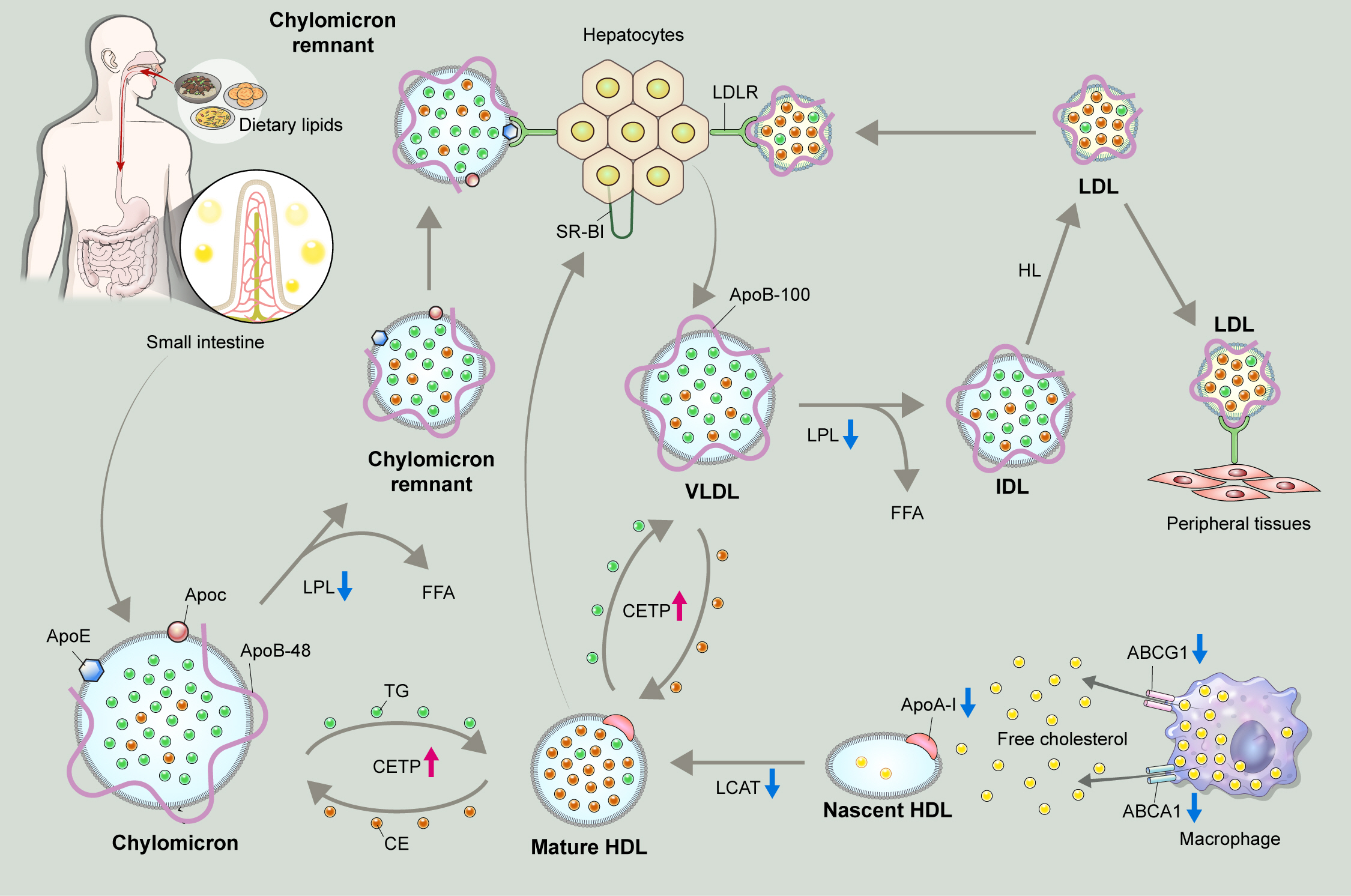
- Dyslipidemia is a potentially modifiable cardiovascular risk factor. Whereas the recommendations for the treatment target of dyslipidemia in the general population are being more and more rigorous, the 2013 Kidney Disease: Improving Global Outcomes clinical practice guideline for lipid management in chronic kidney disease (CKD) presented a relatively conservative approach with respect to the indication of lipid lowering therapy and therapeutic monitoring among the patients with CKD. This may be largely attributed to the lack of high-quality evidence derived from CKD population, among whom the overall feature of dyslipidemia is considerably distinctive to that of general population. In this review article, we cover the characteristic features of dyslipidemia and impact of dyslipidemia on cardiovascular outcomes in patients with CKD. We also review the current evidence on lipid lowering therapy to modify the risk of cardiovascular events in this population. We finally discuss the association between dyslipidemia and CKD progression and the potential strategy to delay the progression of CKD in relation to lipid lowering therapy.
Keyword
Figure
Reference
-
1. Hager MR, Narla AD, Tannock LR. Dyslipidemia in patients with chronic kidney disease. Rev Endocr Metab Disord. 2017; 18:29–40.2. Visconti L, Benvenga S, Lacquaniti A, Cernaro V, Bruzzese A, Conti G, et al. Lipid disorders in patients with renal failure: role in cardiovascular events and progression of chronic kidney disease. J Clin Transl Endocrinol. 2016; 6:8–14.3. Florens N, Calzada C, Lyasko E, Juillard L, Soulage CO. Modified lipids and lipoproteins in chronic kidney disease: a new class of uremic toxins. Toxins (Basel). 2016; 8:376.4. Mesquita J, Varela A, Medina JL. Dyslipidemia in renal disease: causes, consequences and treatment. Endocrinol Nutr. 2010; 57:440–8.5. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007; 115:450–8.6. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease: the Framingham Study. Am J Med. 1977; 62:707–14.7. Arntzenius AC, Kromhout D, Barth JD, Reiber JH, Bruschke AV, Buis B, et al. Diet, lipoproteins, and the progression of coronary atherosclerosis: the Leiden Intervention Trial. N Engl J Med. 1985; 312:805–11.8. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019; 139:e1046–81.9. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020; 41:111–88.10. Anderson TJ, Gregoire J, Pearson GJ, Barry AR, Couture P, Dawes M, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016; 32:1263–82.11. Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014; 85:1303–9.12. Vaziri ND, Sato T, Liang K. Molecular mechanisms of altered cholesterol metabolism in rats with spontaneous focal glomerulosclerosis. Kidney Int. 2003; 63:1756–63.13. Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990; 15:458–82.14. Baigent C, Landray MJ, Wheeler DC. Misleading associations between cholesterol and vascular outcomes in dialysis patients: the need for randomized trials. Semin Dial. 2007; 20:498–503.15. Lee C, Park JT, Chang TI, Kang EW, Nam KH, Joo YS, et al. Low-density lipoprotein cholesterol levels and adverse clinical outcomes in chronic kidney disease: results from the KNOWCKD. Nutr Metab Cardiovasc Dis. 2022; 32:410–9.16. Suh SH, Oh TR, Choi HS, Kim CS, Bae EH, Oh KH, et al. Serum triglycerides level is independently associated with renal outcomes in patients with non-dialysis chronic kidney disease: results from KNOW-CKD study. Front Nutr. 2022; 9:1037618.17. Nam KH, Chang TI, Joo YS, Kim J, Lee S, Lee C, et al. Association between serum high-density lipoprotein cholesterol levels and progression of chronic kidney disease: results from the KNOW-CKD. J Am Heart Assoc. 2019; 8:e011162.18. Suh SH, Oh TR, Choi HS, Kim CS, Bae EH, Ma SK, et al. Non-high-density lipoprotein cholesterol and cardiovascular outcomes in chronic kidney disease: results from KNOW-CKD Study. Nutrients. 2022; 14:3792.19. Suh SH, Oh TR, Choi HS, Kim CS, Bae EH, Ma SK, et al. Nonhigh-density lipoprotein cholesterol and progression of chronic kidney disease: results from the KNOW-CKD Study. Nutrients. 2022; 14:4704.20. Cho SMJ, Lee H, Lee HH, Baek J, Heo JE, Joo HJ, et al. Dyslipidemia fact sheets in Korea 2020: an analysis of nationwide population-based data. J Lipid Atheroscler. 2021; 10:202–9.21. Boren J, Williams KJ. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Curr Opin Lipidol. 2016; 27:473–83.22. Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, et al. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol. 2019; 4:1287–95.23. Reiss AB, Voloshyna I, De Leon J, Miyawaki N, Mattana J. Cholesterol metabolism in CKD. Am J Kidney Dis. 2015; 66:1071–82.24. Chen H, Chen L, Liu D, Chen DQ, Vaziri ND, Yu XY, et al. Combined clinical phenotype and lipidomic analysis reveals the impact of chronic kidney disease on lipid metabolism. J Proteome Res. 2017; 16:1566–78.25. Chu M, Wang AY, Chan IH, Chui SH, Lam CW. Serum small-dense LDL abnormalities in chronic renal disease patients. Br J Biomed Sci. 2012; 69:99–102.26. Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996; 276:875–81.27. Qiao YN, Zou YL, Guo SD. Low-density lipoprotein particles in atherosclerosis. Front Physiol. 2022; 13:931931.28. Kronenberg F. HDL in CKD: the devil is in the detail. J Am Soc Nephrol. 2018; 29:1356–71.29. Batista MC, Welty FK, Diffenderfer MR, Sarnak MJ, Schaefer EJ, Lamon-Fava S, et al. Apolipoprotein A-I, B-100, and B-48 metabolism in subjects with chronic kidney disease, obesity, and the metabolic syndrome. Metabolism. 2004; 53:1255–61.30. Cardinal H, Raymond MA, Hebert MJ, Madore F. Uraemic plasma decreases the expression of ABCA1, ABCG1 and cell-cycle genes in human coronary arterial endothelial cells. Nephrol Dial Transplant. 2007; 22:409–16.31. Calabresi L, Simonelli S, Conca P, Busnach G, Cabibbe M, Gesualdo L, et al. Acquired lecithin:cholesterol acyltransferase deficiency as a major factor in lowering plasma HDL levels in chronic kidney disease. J Intern Med. 2015; 277:552–61.32. Vaziri ND, Liang K, Parks JS. Down-regulation of hepatic lecithin:cholesterol acyltransferase gene expression in chronic renal failure. Kidney Int. 2001; 59:2192–6.33. Seiler S, Schlitt A, Jiang XC, Ulrich C, Blankenberg S, Lackner KJ, et al. Cholesteryl ester transfer protein activity and cardiovascular events in patients with chronic kidney disease stage V. Nephrol Dial Transplant. 2008; 23:3599–604.34. Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, et al. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol. 2012; 60:2372–9.35. Shroff R, Speer T, Colin S, Charakida M, Zewinger S, Staels B, et al. HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype. J Am Soc Nephrol. 2014; 25:2658–68.36. Castillo-Nunez Y, Morales-Villegas E, Aguilar-Salinas CA. Triglyceride-rich lipoproteins: their role in atherosclerosis. Rev Invest Clin. 2022; 74:61–70.37. Duran EK, Pradhan AD. Triglyceride-rich lipoprotein remnants and cardiovascular disease. Clin Chem. 2021; 67:183–96.38. Zhang BH, Yin F, Qiao YN, Guo SD. Triglyceride and triglyceride-rich lipoproteins in atherosclerosis. Front Mol Biosci. 2022; 9:909151.39. de Vries MA, Klop B, Janssen HW, Njo TL, Westerman EM, Castro Cabezas M. Postprandial inflammation: targeting glucose and lipids. Adv Exp Med Biol. 2014; 824:161–70.40. Kwan BC, Kronenberg F, Beddhu S, Cheung AK. Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol. 2007; 18:1246–61.41. Moon JH, Kim K, Choi SH. Lipoprotein lipase: is it a magic target for the treatment of hypertriglyceridemia. Endocrinol Metab (Seoul). 2022; 37:575–86.42. Vaziri ND, Yuan J, Ni Z, Nicholas SB, Norris KC. Lipoprotein lipase deficiency in chronic kidney disease is accompanied by down-regulation of endothelial GPIHBP1 expression. Clin Exp Nephrol. 2012; 16:238–43.43. Vaziri ND, Wang XQ, Liang K. Secondary hyperparathyroidism downregulates lipoprotein lipase expression in chronic renal failure. Am J Physiol. 1997; 273:F925–30.44. Vaziri ND, Liang K. Down-regulation of tissue lipoprotein lipase expression in experimental chronic renal failure. Kidney Int. 1996; 50:1928–35.45. Vaziri ND, Liang K. Down-regulation of VLDL receptor expression in chronic experimental renal failure. Kidney Int. 1997; 51:913–9.46. van Capelleveen JC, van der Valk FM, Stroes ES. Current therapies for lowering lipoprotein (a). J Lipid Res. 2016; 57:1612–8.47. Klezovitch O, Edelstein C, Zhu L, Scanu AM. Apolipoprotein (a) binds via its C-terminal domain to the protein core of the proteoglycan decorin: implications for the retention of lipoprotein (a) in atherosclerotic lesions. J Biol Chem. 1998; 273:23856–65.48. Hopewell JC, Haynes R, Baigent C. The role of lipoprotein (a) in chronic kidney disease. J Lipid Res. 2018; 59:577–85.49. Wang X, Li J, Ju J, Fan Y, Xu H. Effect of different types and dosages of statins on plasma lipoprotein(a) levels: a network meta-analysis. Pharmacol Res. 2021; 163:105275.50. Tsimikas S, Gordts PL, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020; 41:2275–84.51. Bajaj A, Damrauer SM, Anderson AH, Xie D, Budoff MJ, Go AS, et al. Lipoprotein(a) and risk of myocardial infarction and death in chronic kidney disease: findings from the CRIC Study (Chronic Renal Insufficiency Cohort). Arterioscler Thromb Vasc Biol. 2017; 37:1971–8.52. Milionis HJ, Elisaf MS, Tselepis A, Bairaktari E, Karabina SA, Siamopoulos KC. Apolipoprotein(a) phenotypes and lipoprotein (a) concentrations in patients with renal failure. Am J Kidney Dis. 1999; 33:1100–6.53. Sechi LA, Zingaro L, De Carli S, Sechi G, Catena C, Falleti E, et al. Increased serum lipoprotein(a) levels in patients with early renal failure. Ann Intern Med. 1998; 129:457–61.54. Kronenberg F, Trenkwalder E, Lingenhel A, Friedrich G, Lhotta K, Schober M, et al. Renovascular arteriovenous differences in Lp[a] plasma concentrations suggest removal of Lp[a] from the renal circulation. J Lipid Res. 1997; 38:1755–63.55. Reblin T, Donarski N, Fineder L, Brasen JH, Dieplinger H, Thaiss F, et al. Renal handling of human apolipoprotein(a) and its fragments in the rat. Am J Kidney Dis. 2001; 38:619–30.56. Cain WJ, Millar JS, Himebauch AS, Tietge UJ, Maugeais C, Usher D, et al. Lipoprotein [a] is cleared from the plasma primarily by the liver in a process mediated by apolipoprotein [a]. J Lipid Res. 2005; 46:2681–91.57. Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009; 302:1993–2000.58. Saran R, Robinson B, Abbott KC, Agodoa LY, Albertus P, Ayanian J, et al. US renal data system 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017; 69(3 Suppl 1):A7–8.59. Methven S, Steenkamp R, Fraser S. UK Renal Registry 19th annual report: chapter 5 survival and causes of death in UK adult patients on renal replacement therapy in 2015: national and centre-specific analyses. Nephron. 2017; 137 Suppl 1:117–50.60. Cholesterol Treatment Trialists’ (CTT) Collaboration, Herrington WG, Emberson J, Mihaylova B, Blackwell L, Reith C, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 2016; 4:829–39.61. Chue CD, Townend JN, Steeds RP, Ferro CJ. Arterial stiffness in chronic kidney disease: causes and consequences. Heart. 2010; 96:817–23.62. Zanoli L, Lentini P, Briet M, Castellino P, House AA, London GM, et al. Arterial stiffness in the heart disease of CKD. J Am Soc Nephrol. 2019; 30:918–28.63. Edwards NC, Moody WE, Chue CD, Ferro CJ, Townend JN, Steeds RP. Defining the natural history of uremic cardiomyopathy in chronic kidney disease: the role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2014; 7:703–14.64. Mall G, Huther W, Schneider J, Lundin P, Ritz E. Diffuse intermyocardiocytic fibrosis in uraemic patients. Nephrol Dial Transplant. 1990; 5:39–44.65. Aoki J, Ikari Y, Nakajima H, Mori M, Sugimoto T, Hatori M, et al. Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney Int. 2005; 67:333–40.66. Storey BC, Staplin N, Haynes R, Reith C, Emberson J, Herrington WG, et al. Lowering LDL cholesterol reduces cardiovascular risk independently of presence of inflammation. Kidney Int. 2018; 93:1000–7.67. Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007; 357:2109–22.68. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011; 365:2255–67.69. Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012; 367:2089–99.70. HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014; 371:203–12.71. Zewinger S, Speer T, Kleber ME, Scharnagl H, Woitas R, Lepper PM, et al. HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol. 2014; 25:1073–82.72. Silbernagel G, Genser B, Drechsler C, Scharnagl H, Grammer TB, Stojakovic T, et al. HDL cholesterol, apolipoproteins, and cardiovascular risk in hemodialysis patients. J Am Soc Nephrol. 2015; 26:484–92.73. Moradi H, Streja E, Kashyap ML, Vaziri ND, Fonarow GC, Kalantar-Zadeh K. Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol Dial Transplant. 2014; 29:1554–62.74. Kim JY, Park JT, Kim HW, Chang TI, Kang EW, Ahn C, et al. Inflammation alters relationship between high-density lipoprotein cholesterol and cardiovascular risk in patients with chronic kidney disease: results from KNOW-CKD. J Am Heart Assoc. 2021; 10:e021731.75. Levy RI, Glueck CJ. Hypertriglyceridemia, diabetes mellitus, and coronary vessel disease. Arch Intern Med. 1969; 123:220–8.76. Zammit AR, Katz MJ, Derby C, Bitzer M, Lipton RB. Chronic kidney disease in non-diabetic older adults: associated roles of the metabolic syndrome, inflammation, and insulin resistance. PLoS One. 2015; 10:e0139369.77. Soohoo M, Hashemi L, Hsiung JT, Moradi H, Budoff MJ, Kovesdy CP, et al. Risk of atherosclerotic cardiovascular disease and nonatherosclerotic cardiovascular disease hospitalizations for triglycerides across chronic kidney disease stages among 2.9 million US veterans. J Am Heart Assoc. 2021; 10:e022988.78. Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol. 2005; 96(5A):24F–33F.79. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994; 344:1383–9.80. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996; 335:1001–9.81. Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004; 364:685–96.82. Collins R, Armitage J, Parish S, Sleigh P, Peto R; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003; 361:2005–16.83. Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005; 353:238–48.84. Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009; 360:1395–407.85. Marz W, Genser B, Drechsler C, Krane V, Grammer TB, Ritz E, et al. Atorvastatin and low-density lipoprotein cholesterol in type 2 diabetes mellitus patients on hemodialysis. Clin J Am Soc Nephrol. 2011; 6:1316–25.86. Peto R. Current misconception 3: that subgroup-specific trial mortality results often provide a good basis for individualising patient care. Br J Cancer. 2011; 104:1057–8.87. Sleight P. Debate: subgroup analyses in clinical trials: fun to look at - but don’t believe them! Curr Control Trials Cardiovasc Med. 2000; 1:25–7.88. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011; 377:2181–92.89. Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010; 376:1670–81.90. Tonelli M, Muntner P, Lloyd A, Manns B, Klarenbach S, Pannu N, et al. Association between LDL-C and risk of myocardial infarction in CKD. J Am Soc Nephrol. 2013; 24:979–86.91. Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995; 333:1301–7.92. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017; 69:692–711.93. Streja E, Gosmanova EO, Molnar MZ, Soohoo M, Moradi H, Potukuchi PK, et al. Association of continuation of statin therapy initiated before transition to chronic dialysis therapy with mortality after dialysis initiation. JAMA Netw Open. 2018; 1:e182311.94. Rhee EJ, Kim HC, Kim JH, Lee EY, Kim BJ, Kim EM, et al. 2018 Guidelines for the management of dyslipidemia in Korea. J Lipid Atheroscler. 2019; 8:78–131.95. Holdaas H, Fellstrom B, Jardine AG, Holme I, Nyberg G, Fauchald P, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003; 361:2024–31.96. Holdaas H, Fellstrom B, Cole E, Nyberg G, Olsson AG, Pedersen TR, et al. Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am J Transplant. 2005; 5:2929–36.97. Jun M, Zhu B, Tonelli M, Jardine MJ, Patel A, Neal B, et al. Effects of fibrates in kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2012; 60:2061–71.98. Hadjivasilis A, Kouis P, Kousios A, Panayiotou A. The effect of fibrates on kidney function and chronic kidney disease progression: a systematic review and meta-analysis of randomised studies. J Clin Med. 2022; 11:768.99. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019; 380:11–22.100. Majithia A, Bhatt DL, Friedman AN, Miller M, Steg PG, Brinton EA, et al. Benefits of icosapent ethyl across the range of kidney function in patients with established cardiovascular disease or diabetes: REDUCE-IT RENAL. Circulation. 2021; 144:1750–9.101. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH Randomized Clinical Trial. JAMA. 2020; 324:2268–80.102. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020; 41:111–88.103. Marais DA, Blom DJ, Petrides F, Goueffic Y, Lambert G. Proprotein convertase subtilisin/kexin type 9 inhibition. Curr Opin Lipidol. 2012; 23:511–7.104. Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J Lipid Res. 2012; 53:2515–24.105. Colhoun HM, Robinson JG, Farnier M, Cariou B, Blom D, Kereiakes DJ, et al. Efficacy and safety of alirocumab, a fully human PCSK9 monoclonal antibody, in high cardiovascular risk patients with poorly controlled hypercholesterolemia on maximally tolerated doses of statins: rationale and design of the ODYSSEY COMBO I and II trials. BMC Cardiovasc Disord. 2014; 14:121.106. Kastelein JJ, Robinson JG, Farnier M, Krempf M, Langslet G, Lorenzato C, et al. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia not adequately controlled with current lipid-lowering therapy: design and rationale of the ODYSSEY FH studies. Cardiovasc Drugs Ther. 2014; 28:281–9.107. Moriarty PM, Jacobson TA, Bruckert E, Thompson PD, Guyton JR, Baccara-Dinet MT, et al. Efficacy and safety of alirocumab, a monoclonal antibody to PCSK9, in statin-intolerant patients: design and rationale of ODYSSEY ALTERNATIVE, a randomized phase 3 trial. J Clin Lipidol. 2014; 8:554–61.108. Robinson JG, Colhoun HM, Bays HE, Jones PH, Du Y, Hanotin C, et al. Efficacy and safety of alirocumab as add-on therapy in high-cardiovascular-risk patients with hypercholesterolemia not adequately controlled with atorvastatin (20 or 40 mg) or rosuvastatin (10 or 20 mg): design and rationale of the ODYSSEY OPTIONS Studies. Clin Cardiol. 2014; 37:597–604.109. Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014; 168:682–9.110. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015; 36:1186–94.111. Bays H, Gaudet D, Weiss R, Ruiz JL, Watts GF, Gouni-Berthold I, et al. Alirocumab as add-on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab. 2015; 100:3140–8.112. Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015; 36:2996–3003.113. Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J. 2015; 169:906–15.114. Roth EM, McKenney JM. ODYSSEY MONO: effect of alirocumab 75 mg subcutaneously every 2 weeks as monotherapy versus ezetimibe over 24 weeks. Future Cardiol. 2015; 11:27–37.115. Farnier M, Jones P, Severance R, Averna M, Steinhagen-Thiessen E, Colhoun HM, et al. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016; 244:138–46.116. Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014; 311:1870–82.117. Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014; 63:2541–8.118. Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014; 63:2531–40.119. Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014; 370:1809–19.120. Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015; 385:331–40.121. Toth PP, Dwyer JP, Cannon CP, Colhoun HM, Rader DJ, Upadhyay A, et al. Efficacy and safety of lipid lowering by alirocumab in chronic kidney disease. Kidney Int. 2018; 93:1397–408.122. Charytan DM, Sabatine MS, Pedersen TR, Im K, Park JG, Pineda AL, et al. Efficacy and safety of evolocumab in chronic kidney disease in the FOURIER Trial. J Am Coll Cardiol. 2019; 73:2961–70.123. Haynes R, Lewis D, Emberson J, Reith C, Agodoa L, Cass A, et al. Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol. 2014; 25:1825–33.124. Su X, Zhang L, Lv J, Wang J, Hou W, Xie X, et al. Effect of statins on kidney disease outcomes: a systematic review and meta-analysis. Am J Kidney Dis. 2016; 67:881–92.125. Jhee JH, Joo YS, Park JT, Yoo TH, Park SK, Jung JY, et al. Intensity of statin therapy and renal outcome in chronic kidney disease: results from the Korean cohort study for outcome in patients with chronic kidney disease. Kidney Res Clin Pract. 2020; 39:93–102.126. de Boer IH, Zelnick LR, Ruzinski J, Friedenberg G, Duszlak J, Bubes VY, et al. Effect of vitamin D and omega-3 fatty acid supplementation on kidney function in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2019; 322:1899–909.127. Yen CL, Fan PC, Lin MS, Lee CC, Tu KH, Chen CY, et al. Fenofibrate delays the need for dialysis and reduces cardiovascular risk among patients with advanced CKD. J Clin Endocrinol Metab. 2021; 106:1594–605.128. Seki M, Nakano T, Tanaka S, Matsukuma Y, Funakoshi K, Ohkuma T, et al. Design and methods of an open-label, randomized controlled trial to evaluate the effect of pemafibrate on proteinuria in CKD patients (PROFIT-CKD). Clin Exp Nephrol. 2023; 27:358–64.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- T helper 17 cells in the pathophysiology of acute and chronic kidney disease
- Post-Renal Transplantation Dyslipidemia
- Immunologic Aspects of Dyslipidemia: a Critical Regulator of Adaptive Immunity and Immune Disorders
- New European Society of Cardiology/European Atherosclerosis Society Guideline for the Management of Dyslipidemia
- Mechanism, clinical consequences, and management of dyslipidemia in children with nephrotic syndrome