Int J Stem Cells.
2023 Aug;16(3):326-341. 10.15283/ijsc22046.
Wedelolactone Promotes the Chondrogenic Differentiation of Mesenchymal Stem Cells by Suppressing EZH2
- Affiliations
-
- 1Research Center for Integrative Medicine, School of Basic Medical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Traditional Chinese Medicine Innovation Research Center, Shenzhen Hospital of Integrated Traditional Chinese and Western Medicine, Shenzhen, China
- 3Guangdong Key Laboratory of Liver Disease Research, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
- KMID: 2545232
- DOI: http://doi.org/10.15283/ijsc22046
Abstract
- Background and Objectives
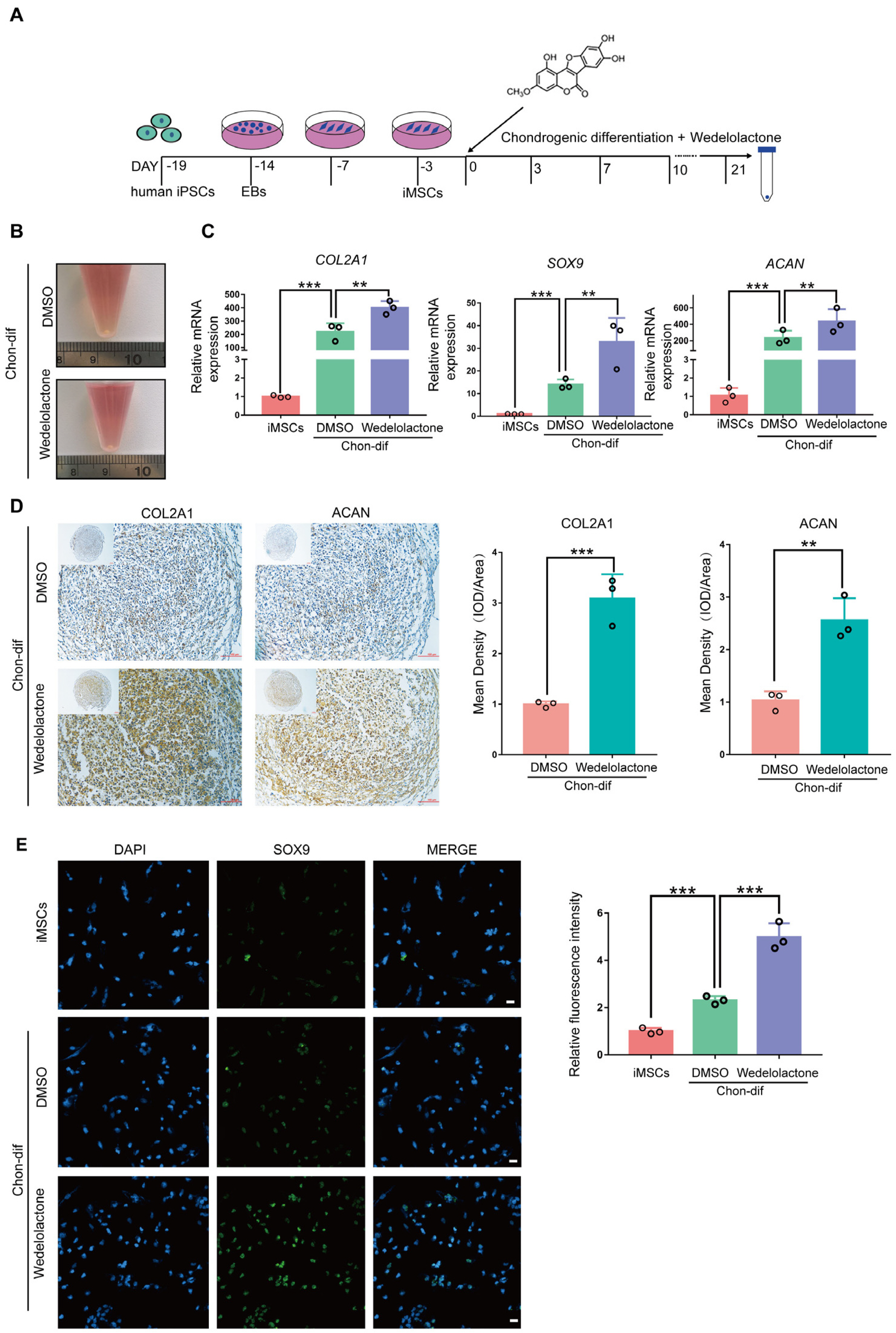
Osteoarthritis (OA) is a degenerative disease that leads to the progressive destruction of articular cartilage. Current clinical therapeutic strategies are moderately effective at relieving OA-associated pain but cannot induce chondrocyte differentiation or achieve cartilage regeneration. We investigated the ability of wedelolactone, a biologically active natural product that occurs in Eclipta alba (false daisy), to promote chondrogenic differentiation.
Methods and Results
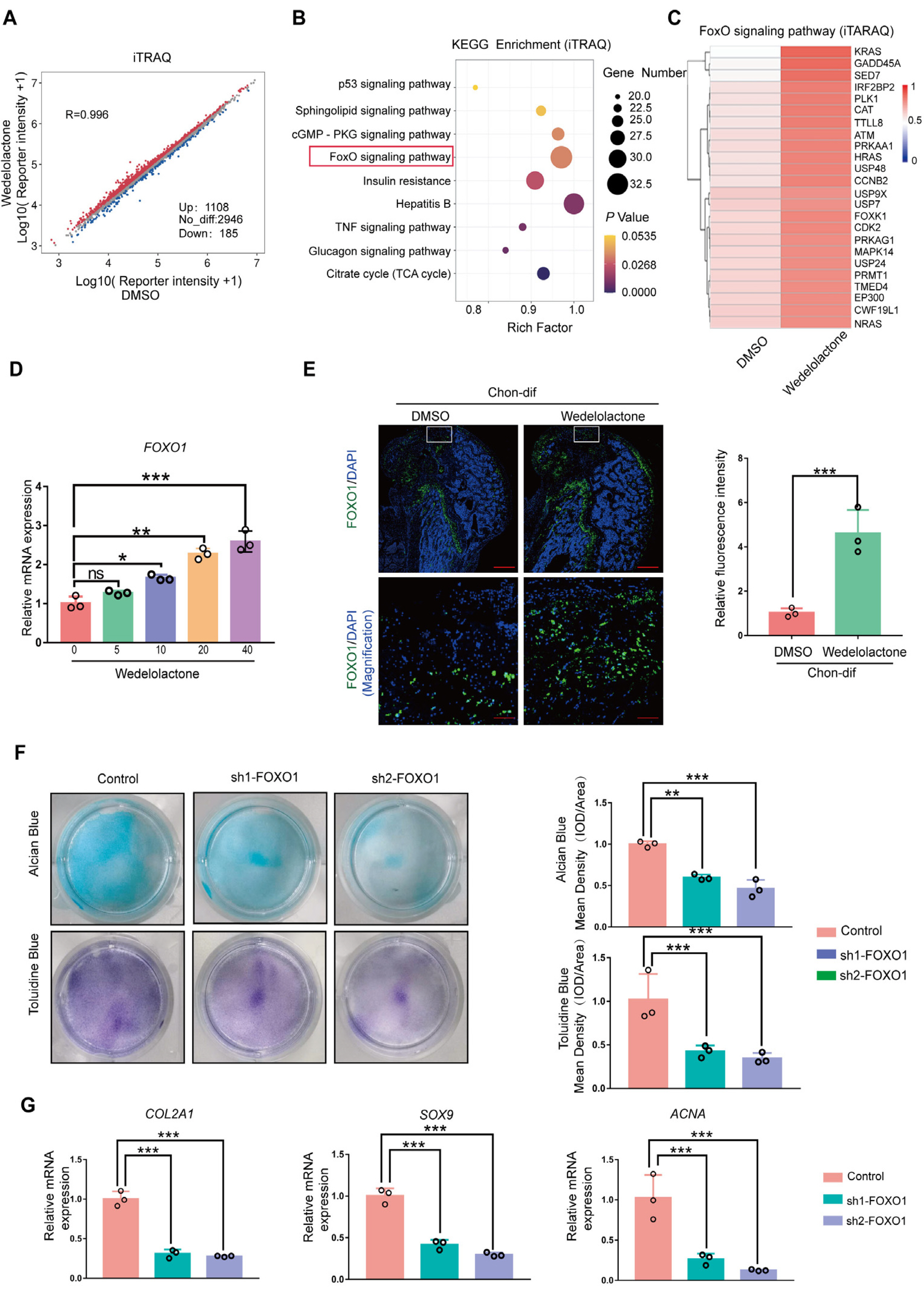
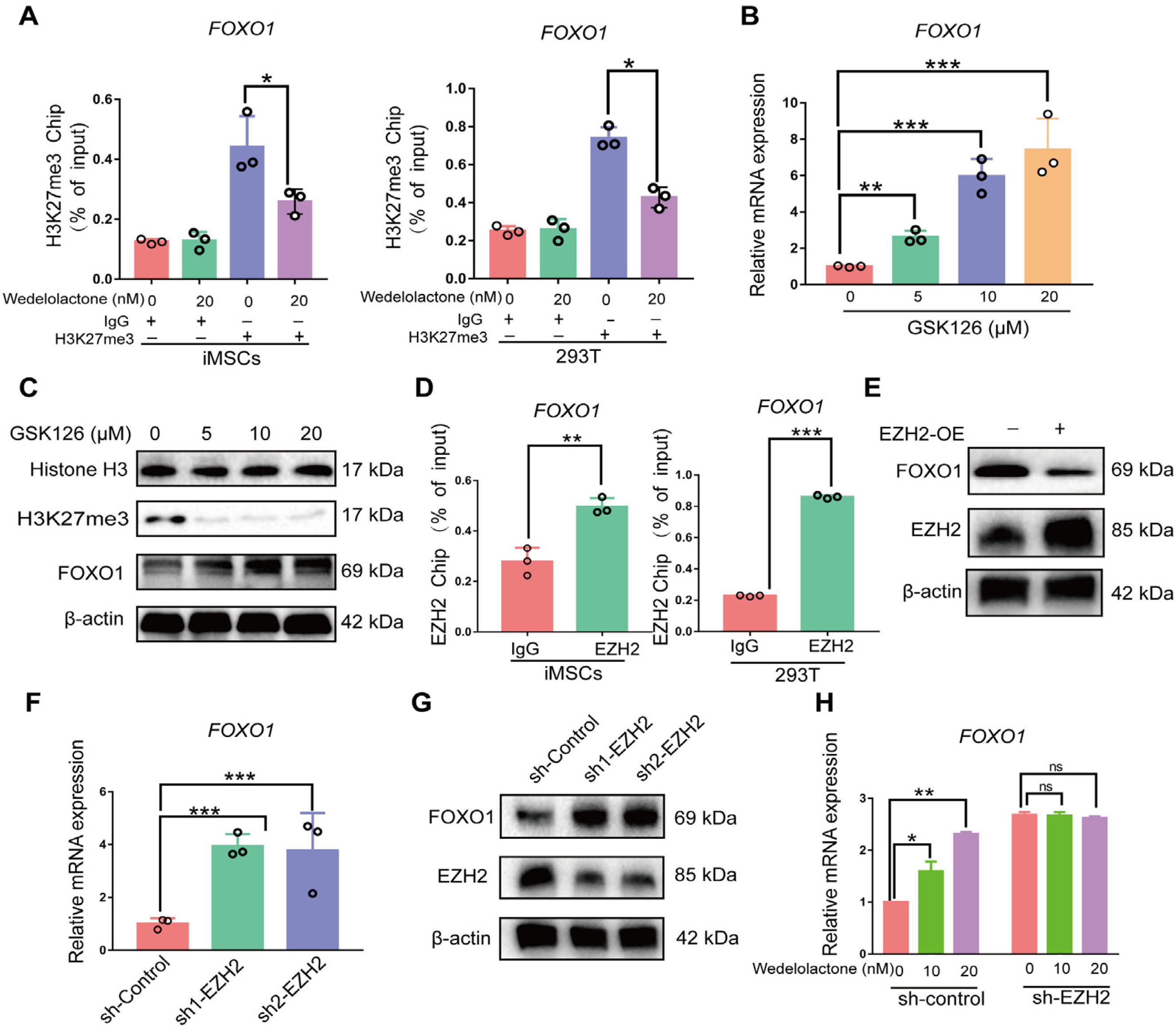
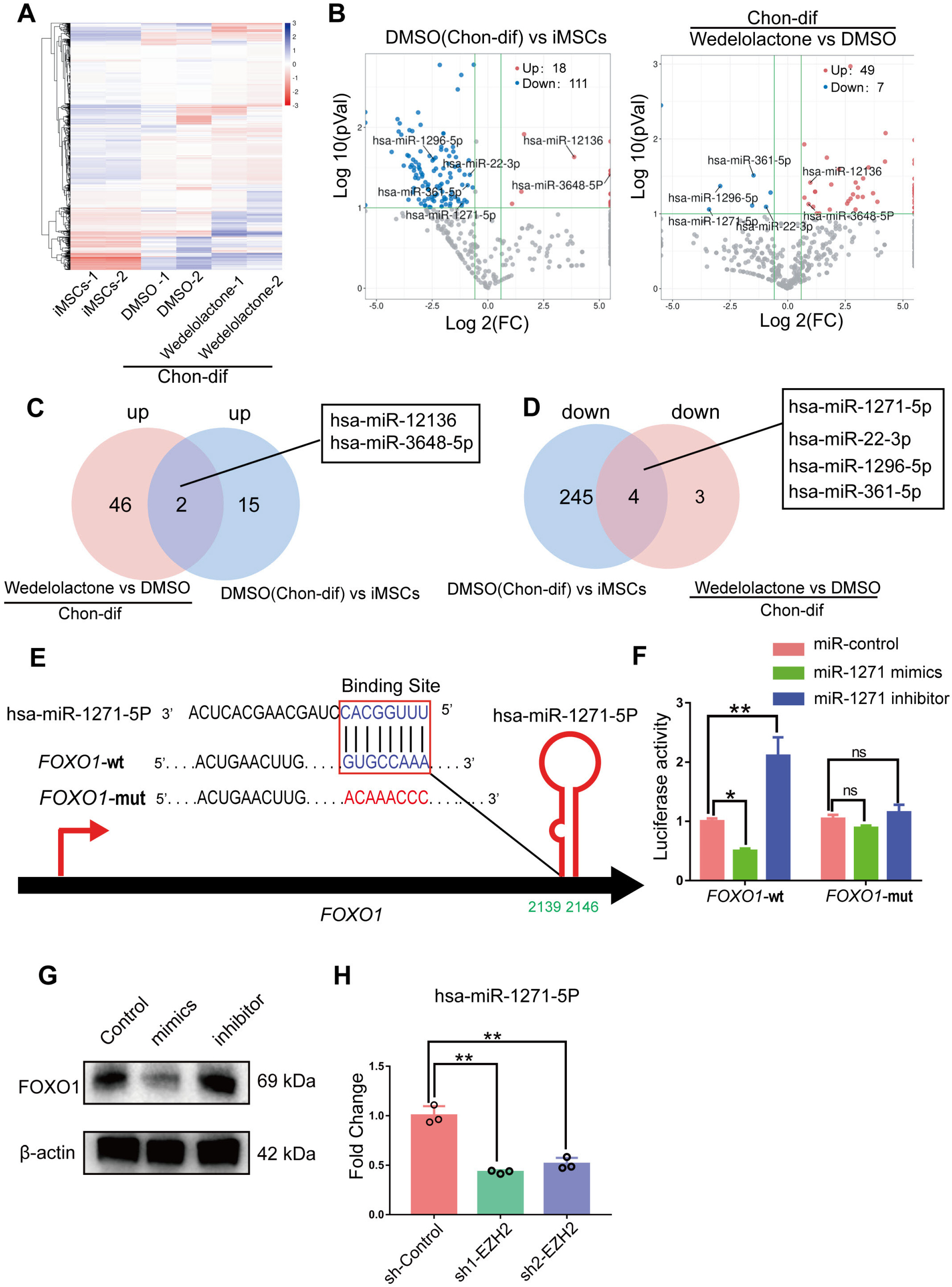
Real-time reverse transcription–polymerase chain reaction, immunohistochemical staining, and immunofluorescence staining assays were used to evaluate the effects of wedelolactone on the chondrogenic differentiation of mesenchymal stem cells (MSCs). RNA sequencing, microRNA (miRNA) sequencing, and isobaric tags for relative and absolute quantitation analyses were performed to explore the mechanism by which wedelolactone promotes the chondrogenic differentiation of MSCs. We found that wedelolactone facilitates the chondrogenic differentiation of human induced pluripotent stem cell-derived MSCs and rat bone-marrow MSCs. Moreover, the forkhead box O (FOXO) signaling pathway was upregulated by wedelolactone during chondrogenic differentiation, and a FOXO1 inhibitor attenuated the effect of wedelolactone on chondrocyte differentiation. We determined that wedelolactone reduces enhancer of zeste homolog 2 (EZH2)-mediated histone H3 lysine 27 trimethylation of the promoter region of FOXO1 to upregulate its transcription. Additionally, we found that wedelolactone represses miR-1271-5p expression, and that miR-1271-5p post-transcriptionally suppresses the expression of FOXO1 that is dependent on the binding of miR-1271-5p to the FOXO1 3’-untranscribed region.
Conclusions
These results indicate that wedelolactone suppresses the activity of EZH2 to facilitate the chondrogenic differentiation of MSCs by activating the FOXO1 signaling pathway. Wedelolactone may therefore improve cartilage regeneration in diseases characterized by inflammatory tissue destruction, such as OA.
Keyword
Figure
Reference
-
References
1. Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, Goldring SR, Jones G, Teichtahl AJ, Pelletier JP. 2016; Osteoarthritis. Nat Rev Dis Primers. 2:16072. DOI: 10.1038/nrdp.2016.72. PMID: 27734845.
Article2. Xie J, Wang Y, Lu L, Liu L, Yu X, Pei F. 2021; Cellular senescence in knee osteoarthritis: molecular mechanisms and therapeutic implications. Ageing Res Rev. 70:101413. DOI: 10.1016/j.arr.2021.101413. PMID: 34298194.
Article3. Jiang Y, Tuan RS. 2015; Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 11:206–212. DOI: 10.1038/nrrheum.2014.200. PMID: 25536487. PMCID: PMC5413931.
Article4. Zhu MM, Wang L, Yang D, Li C, Pang ST, Li XH, Li R, Yang B, Lian YP, Ma L, Lv QL, Jia XB, Feng L. 2019; Wedelolactone alleviates doxorubicin-induced inflamma-tion and oxidative stress damage of podocytes by IκK/IκB/NF-κB pathway. Biomed Pharmacother. 117:109088. DOI: 10.1016/j.biopha.2019.109088. PMID: 31202173.
Article5. Pan H, Lin Y, Dou J, Fu Z, Yao Y, Ye S, Zhang S, Wang N, Liu A, Li X, Zhang F, Chen D. 2020; Wedelolactone facilitates Ser/Thr phosphorylation of NLRP3 dependent on PKA signalling to block inflammasome activation and py-roptosis. Cell Prolif. 53:e12868. DOI: 10.1111/cpr.12868. PMID: 32656909. PMCID: PMC7507381.
Article6. Lim S, Jang HJ, Park EH, Kim JK, Kim JM, Kim EK, Yea K, Kim YH, Lee-Kwon W, Ryu SH, Suh PG. 2012; Wedelolactone inhibits adipogenesis through the ERK pathway in human adipose tissue-derived mesenchymal stem cells. J Cell Bioc-hem. 113:3436–3445. DOI: 10.1002/jcb.24220. PMID: 22678810.
Article7. Liu YQ, Han XF, Bo JX, Ma HP. 2016; Wedelolactone enhances osteoblastogenesis but inhibits osteoclastogenesis through Sema3A/NRP1/PlexinA1 pathway. Front Pharmacol. 7:375. DOI: 10.3389/fphar.2016.00375. PMID: 27803667. PMCID: PMC5067540. PMID: a4d67c3b03bb40008dae9f889899b86b.
Article8. Liu YQ, Hong ZL, Zhan LB, Chu HY, Zhang XZ, Li GH. 2016; Wedelolactone enhances osteoblastogenesis by regulating Wnt/β-catenin signaling pathway but suppresses osteoclastogenesis by NF-κB/c-fos/NFATc1 pathway. Sci Rep. 6:32260. DOI: 10.1038/srep32260. PMID: 27558652. PMCID: PMC4997609.
Article9. Deng X, Liang LN, Zhu D, Zheng LP, Yu JH, Meng XL, Zhao YN, Sun XX, Pan TW, Liu YQ. 2018; Wedelolactone inhibits osteoclastogenesis but enhances osteoblastogenesis through altering different semaphorins production. Int Immunopharmacol. 60:41–49. DOI: 10.1016/j.intimp.2018.04.037. PMID: 29702282.
Article10. Wang C, Song Y, Gu Z, Lian M, Huang D, Lu X, Feng X, Lu Q. 2018; Wedelolactone enhances odontoblast differentiation by promoting Wnt/β-catenin signaling pathway and suppressing NF-κB signaling pathway. Cell Reprogram. 20:236–244. DOI: 10.1089/cell.2018.0004. PMID: 30089027.
Article11. Idris AI, Libouban H, Nyangoga H, Landao-Bassonga E, Chappard D, Ralston SH. 2009; Pharmacologic inhibitors of IkappaB kinase suppress growth and migration of mammary carcinosarcoma cells in vitro and prevent osteolytic bone metastasis in vivo. Mol Cancer Ther. 8:2339–2347. DOI: 10.1158/1535-7163.MCT-09-0133. PMID: 19671767.
Article12. Xu S, Liu X, Liu X, Shi Y, Jin X, Zhang N, Li X, Zhang H. 2021; Wedelolactone ameliorates Pseudomonas aeruginosa-induced inflammation and corneal injury by suppressing caspase-4/5/11/GSDMD-mediated non-canonical pyroptosis. Exp Eye Res. 211:108750. DOI: 10.1016/j.exer.2021.108750. PMID: 34481822.
Article13. Nehybova T, Smarda J, Daniel L, Brezovsky J, Benes P. 2015; Wedelolactone induces growth of breast cancer cells by stimulation of estrogen receptor signalling. J Steroid Biochem Mol Biol. 152:76–83. DOI: 10.1016/j.jsbmb.2015.04.019. PMID: 25934092.
Article14. Benes P, Knopfova L, Trcka F, Nemajerova A, Pinheiro D, Soucek K, Fojta M, Smarda J. 2011; Inhibition of topoisomerase IIα: novel function of wedelolactone. Cancer Lett. 303:29–38. DOI: 10.1016/j.canlet.2011.01.002. PMID: 21315506.
Article15. Deng H, Fang Y. 2012; Anti-inflammatory gallic Acid and wedelolactone are G protein-coupled receptor-35 agonists. Phar-macology. 89:211–219. DOI: 10.1159/000337184. PMID: 22488351.
Article16. Romanchikova N, Trapencieris P. 2019; Wedelolactone targets EZH2-mediated histone H3K27 methylation in mantle cell lymphoma. Anticancer Res. 39:4179–4184. DOI: 10.21873/anticanres.13577. PMID: 31366503.
Article17. Takahashi K, Yamanaka S. 2006; Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126:663–676. DOI: 10.1016/j.cell.2006.07.024. PMID: 16904174.
Article18. Nam Y, Rim YA, Ju JH. 2017; Chondrogenic pellet formation from cord blood-derived induced pluripotent stem cells. J Vis Exp. (124):55988. DOI: 10.3791/55988. PMID: 28654049. PMCID: PMC5608467.
Article19. Li Y, Hai Y, Chen J, Liu T. 2017; Differentiating chondrocytes from peripheral blood-derived human induced pluripotent stem cells. J Vis Exp. (125):55722. DOI: 10.3791/55722-v. PMID: 28745632. PMCID: PMC5612544.
Article20. Bi R, Yin Q, Mei J, Chen K, Luo X, Fan Y, Zhu S. 2020; Identification of human temporomandibular joint fibrocar-tilage stem cells with distinct chondrogenic capacity. Osteo-arthritis Cartilage. 28:842–852. DOI: 10.1016/j.joca.2020.02.835. PMID: 32147536.
Article21. Xu M, Stattin EL, Shaw G, Heinegård D, Sullivan G, Wilmut I, Colman A, Önnerfjord P, Khabut A, Aspberg A, Dockery P, Hardingham T, Murphy M, Barry F. 2016; Chon-drocytes derived from mesenchymal stromal cells and induced pluripotent cells of patients with familial osteochondritis dissecans exhibit an endoplasmic reticulum stress response and defective matrix assembly. Stem Cells Transl Med. 5:1171–1181. DOI: 10.5966/sctm.2015-0384. PMID: 27388238. PMCID: PMC4996445. PMID: f921e2842d114179b88d89856f7577c0.
Article22. Zhu D, Deng X, Han XF, Sun XX, Pan TW, Zheng LP, Liu YQ. 2018; Wedelolactone enhances osteoblastogenesis through ERK- and JNK-mediated BMP2 expression and Smad/1/5/8 phosphorylation. Molecules. 23:561. DOI: 10.3390/molecules23030561. PMID: 29498687. PMCID: PMC6017959.
Article23. Soh R, Hardy A, Zur Nieden NI. 2021; The FOXO signaling axis displays conjoined functions in redox homeostasis and stemness. Free Radic Biol Med. 169:224–237. DOI: 10.1016/j.freeradbiomed.2021.04.022. PMID: 33878426. PMCID: PMC9910585.
Article24. Akiyama H. 2008; Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol. 18:213–219. DOI: 10.3109/s10165-008-0048-x. PMID: 18351289.
Article25. Cao R, Zhang Y. 2004; The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 14:155–164. DOI: 10.1016/j.gde.2004.02.001. PMID: 15196462.
Article26. Jiang C, Guo Q, Jin Y, Xu JJ, Sun ZM, Zhu DC, Lin JH, Tian NF, Sun LJ, Zhang XL, Wu YS. 2019; Inhibition of EZH2 ameliorates cartilage endplate degeneration and attenuates the progression of intervertebral disc degeneration via demethylation of Sox-9. EBioMedicine. 48:619–629. DOI: 10.1016/j.ebiom.2019.10.006. PMID: 31631036. PMCID: PMC6838408.
Article27. Ludikhuize MC, Meerlo M, Gallego MP, Xanthakis D, Burgaya Julià M, Nguyen NTB, Brombacher EC, Liv N, Maurice MM, Paik JH, Burgering BMT, Rodriguez Colman MJ. 2020; Mitochondria define intestinal stem cell differentiation downstream of a FOXO/Notch axis. Cell Metab. 32:889–900.e7. DOI: 10.1016/j.cmet.2020.10.005. PMID: 33147486.
Article28. Kim DY, Hwang I, Muller FL, Paik JH. 2015; Functional regulation of FoxO1 in neural stem cell differentiation. Cell Death Differ. 22:2034–2045. DOI: 10.1038/cdd.2015.123. PMID: 26470727. PMCID: PMC4816100.
Article29. Guérit D, Brondello JM, Chuchana P, Philipot D, Toupet K, Bony C, Jorgensen C, Noël D. 2014; FOXO3A regulation by miRNA-29a controls chondrogenic differentiation of mesenchymal stem cells and cartilage formation. Stem Cells Dev. 23:1195–1205. DOI: 10.1089/scd.2013.0463. PMID: 24467486.
Article30. Djouad F, Bony C, Canovas F, Fromigué O, Rème T, Jorgensen C, Noël D. 2009; Transcriptomic analysis identifies Foxo3A as a novel transcription factor regulating mesenchymal stem cell chrondrogenic differentiation. Cloning Stem Cells. 11:407–416. DOI: 10.1089/clo.2009.0013. PMID: 19751111.
Article31. Kurakazu I, Akasaki Y, Hayashida M, Tsushima H, Goto N, Sueishi T, Toya M, Kuwahara M, Okazaki K, Duffy T, Lotz MK, Nakashima Y. 2019; FOXO1 transcription factor regulates chondrogenic differentiation through transforming growth factor β1 signaling. J Biol Chem. 294:17555–17569. DOI: 10.1074/jbc.RA119.009409. PMID: 31601652. PMCID: PMC6873195.
Article32. Zheng M, Cao MX, Luo XJ, Li L, Wang K, Wang SS, Wang HF, Tang YJ, Tang YL, Liang XH. 2019; EZH2 promotes invasion and tumour glycolysis by regulating STAT3 and FoxO1 signalling in human OSCC cells. J Cell Mol Med. 23:6942–6954. DOI: 10.1111/jcmm.14579. PMID: 31368152. PMCID: PMC6787444.
Article33. Yu Y, Deng P, Yu B, Szymanski JM, Aghaloo T, Hong C, Wang CY. 2017; Inhibition of EZH2 promotes human embryonic stem cell differentiation into mesoderm by reducing H3K-27me3. Stem Cell Reports. 9:752–761. Erratum in: Stem Cell Reports 2018;11:1579-1580. DOI: 10.1016/j.stemcr.2018.11.013. PMID: 30540964. PMCID: PMC6294283.
Article34. Rougeot J, Chrispijn ND, Aben M, Elurbe DM, Andralojc KM, Murphy PJ, Jansen PWTC, Vermeulen M, Cairns BR, Kamminga LM. 2019; Maintenance of spatial gene expression by Polycomb-mediated repression after formation of a vertebrate body plan. Development. 146:dev178590. DOI: 10.1242/dev.178590. PMID: 31488564. PMCID: PMC6803366.
Article35. Camilleri ET, Dudakovic A, Riester SM, Galeano-Garces C, Paradise CR, Bradley EW, McGee-Lawrence ME, Im HJ, Karperien M, Krych AJ, Westendorf JJ, Larson AN, van Wijnen AJ. 2018; Loss of histone methyltransferase Ezh2 stimulates an osteogenic transcriptional program in chondrocytes but does not affect cartilage development. J Biol Chem. 293:19001–19011. DOI: 10.1074/jbc.RA118.003909. PMID: 30327434. PMCID: PMC6295726.
Article36. Chen L, Wu Y, Wu Y, Wang Y, Sun L, Li F. 2016; The inhibition of EZH2 ameliorates osteoarthritis development through the Wnt/β-catenin pathway. Sci Rep. 6:29176. DOI: 10.1038/srep29176. PMID: 27539752. PMCID: PMC4990905.
Article37. Li F, Chen S, Yu J, Gao Z, Sun Z, Yi Y, Long T, Zhang C, Li Y, Pan Y, Qin C, Long W, Liu Q, Zhao W. 2021; Interplay of m6 A and histone modifications contributes to temozolomide resistance in glioblastoma. Clin Transl Med. 11:e553. DOI: 10.1002/ctm2.553. PMID: 7854cee962c142b590ebd42800c8d82d.
Article38. Asenjo HG, Gallardo A, López-Onieva L, Tejada I, Martorell-Marugán J, Carmona-Sáez P, Landeira D. 2020; Poly-comb regulation is coupled to cell cycle transition in pluripotent stem cells. Sci Adv. 6:eaay4768. DOI: 10.1126/sciadv.aay4768. PMID: 32181346. PMCID: PMC7056320.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fibrin Scaffolds Designing in order to Human Adipose-derived Mesenchymal Stem Cells Differentiation to Chondrocytes in the Presence of TGF-beta3
- Difference in HLA-DR Expression of Human Umbilical Cord Blood Derived Mesenchymal Stem Cells after Tri-lineage Differentiation
- Long Term Exposure to Myrtucommulone-A Changes CD105 Expression and Differentiation Potential of Mesenchymal Stem Cells
- Effects of TGF- beta 3 pretreatment in vitro on the differentiation of rabbit mesenchymal stem cell in vivo
- Hypoxia Pretreatment Promotes Chondrocyte Differentiation of Human Adipose-Derived Stem Cells via Vascular Endothelial Growth Factor