Healthc Inform Res.
2023 Jul;29(3):199-208. 10.4258/hir.2023.29.3.199.
Secondary Use Provisions in the European Health Data Space Proposal and Policy Recommendations for Korea
- Affiliations
-
- 1Ewha Law School, Seoul, Korea
- 2Covington & Burling LLP, Brussels, Belgium
- KMID: 2544871
- DOI: http://doi.org/10.4258/hir.2023.29.3.199
Abstract
Objectives
This article explores the secondary use provisions of the European Health Data Space (EHDS), proposed by the European Commission in May 2022, and offers policy recommendations for South Korea.
Methods
The authors analyzed the texts of the EHDS proposal and other documents published by the European Union, as well as surveyed the relevant literature.
Results
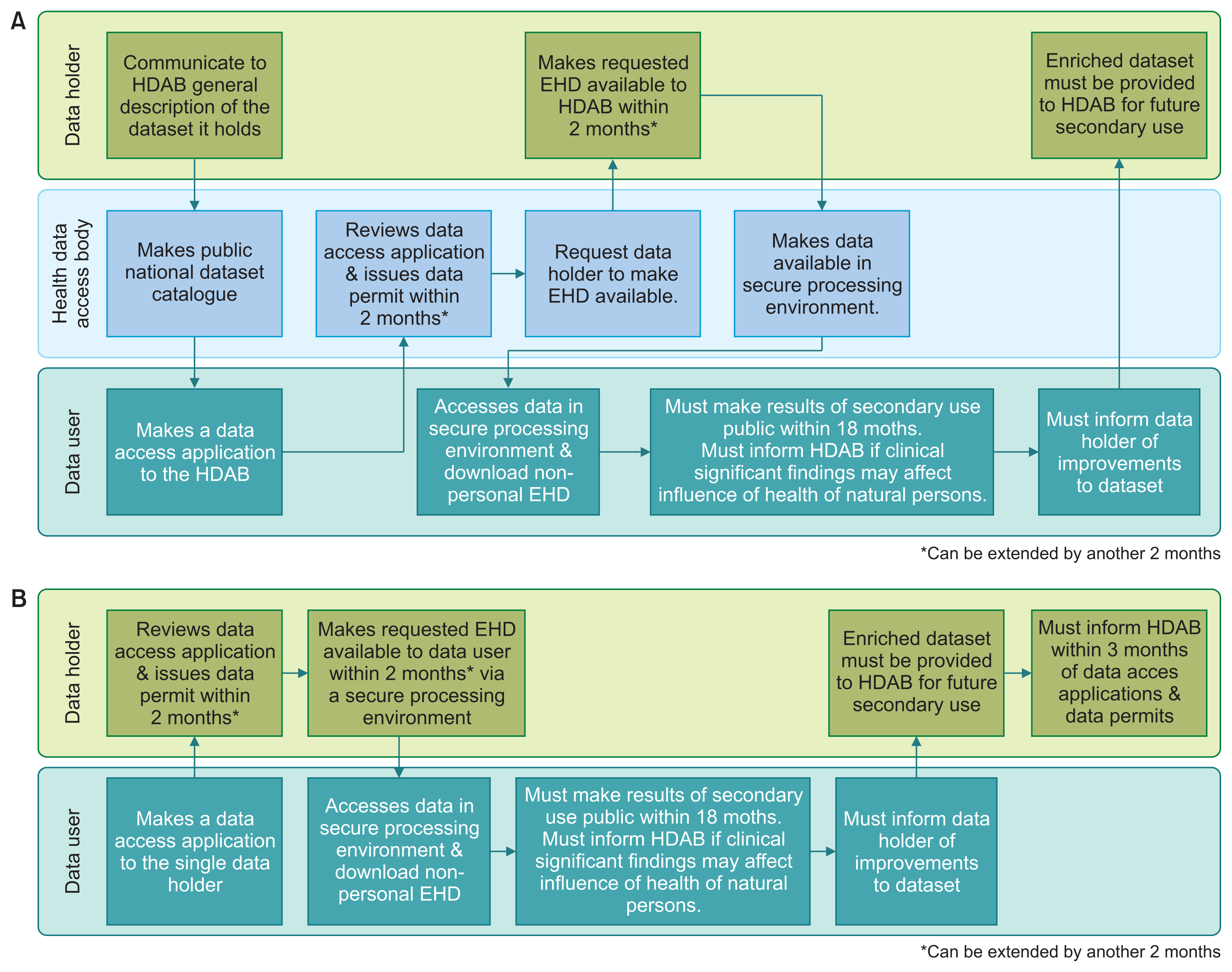
The EHDS proposal seeks to create new patient rights over electronic health data collected and used for primary care; and establish a data sharing system for the re-use of electronic health data for secondary purposes, including research, the provision of personalized healthcare, and developing healthcare artificial intelligence (AI) applications. These provisions envisage requiring both private and public data holders to share certain types of electronic health data on a mandatory basis with third parties. New government bodies, called health data access bodies, would review data access applications and issue data permits.
Conclusions
The overarching aim of the EHDS proposal is to make electronic health data, which are currently held in the hands of a small number of organizations, available for re-use by third parties to stimulate innovation and research. While it will be very challenging for South Korea to adopt a similar scheme and require private entities to share their proprietary data with third parties, the South Korean government should consider making at least health data collected through publicly funded research more readily available for secondary use.
Figure
Reference
-
References
1. European Commission. Proposal for a regulation of the european parliament and of the council on the European health data space [Internet]. Strasbourg, France: European Commission;2022. [cited at 2023 Jul 31]. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52022PC0197.2. European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: a European strategy for data [Internet]. Brussels, Belgium: European Commission;2020. [cited at 2023 Jul 31]. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0066.3. Carvalho G, Kazim E. Themes in data strategy: thematic analysis of ‘A European Strategy for Data’ (EC). AI Ethics. 2022; 2:53–63. https://doi.org/10.1007/s43681-021-00102-y.
Article4. Jauregui AE. Overview of the European Health Data Space (EHDS): goals and current challenges. Eur J Public Health. 2021. 31(Suppl S3):ckab164–123. https://doi.org/10.1093/eurpub/ckab164.123.
Article5. Lee J, Park YT, Park YR, Lee JH. Review of national-level personal health records in advanced countries. Healthc Inform Res. 2021; 27(2):102–9. https://doi.org/10.4258/hir.2021.27.2.102.
Article6. Stellmach C, Muzoora MR, Thun S. Digitalization of health data: interoperability of the proposed European health data space. Stud Health Technol Inform. 2022; 298:132–6. https://doi.org/10.3233/shti220922.
Article7. Das M. Stakeholders welcome proposal on the European Health Data Space. Lancet Oncol. 2022; 23(12):1492. https://doi.org/10.1016/s1470-2045(22)00691-x.
Article8. Horgan D, Hajduch M, Vrana M, Soderberg J, Hughes N, Omar MI, et al. European Health Data Space: an opportunity now to grasp the future of data-driven healthcare. Healthcare (Basel). 2022; 10(9):1629. https://doi.org/10.3390/healthcare10091629.
Article9. Yakovleva S. On digital sovereignty, new European data rules, and the future of free data flows. Legal Iss Econ Integr. 2022; 49(4):339–48.10. Standing Committee of European Doctors (CPME). Position on the European Health Data Space [Internet]. Brussels, Belgium: CPME;2022. [cited at 2023 Jul 31]. Available from: https://www.cpme.eu/api/documents/adopted/2022/11/cpme.2022-065.FINAL.CPME.position.EHDS.pdf.11. Karacic J. Europe, we have a problem! Challenges to health data-sharing in the EU. In : Proceedings of the 2022, 18th International Conference on Wireless and Mobile Computing, Networking and Communications (WiMob); 2022 Oct 10–12; Thessaloniki, Greece. 47–50. https://doi.org/10.1109/wimob55322.2022.9941532.
Article12. Slokenberga S, Tzortzatou O, Reichel J. GDPR and bio-banking: individual rights, public interest and research regulation across Europe. Cham, Switzerland: Springer;2021. 397–419. https://doi.org/10.1007/978-3-030-49388-2.
Article13. Lee SE. The Amendment of Personal Information Protection Act of Korea: focusing on the grounds for processing personal data without data subject’s consent. Ewha Law J. 2020; 24(3):249–86. https://doi.org/10.32632/elj.2020.24.3.249.
Article14. Park YG. Study on the trends of the supreme court cases regarding trade secret protection. J Ind Prop. 2016; 49:231–77.15. Chung KH. Die Begriffe von ‘Enteignug, Nutzung, Beschränkung’ im Sinne von Art. 23 Abs. 3 KV. Const Law. 2019; 25(3):133–87. https://doi.org/10.35901/kjcl.2019.25.3.133.
Article16. Eom T. Policy consideration on the utilization of compulsory license of pharmaceutical patent for overcoming COVID-19. J Intellect Prop. 2022; 17(1):1–40. https://doi.org/10.34122/jip.2022.17.1.1.
Article17. Kim M. A review of legislation related to science and technology research, industry convergence promotion: focused on the latest issues of the special Act on the Innovation of National R&D and Industrial Convergence Promotion Act. J Sci Technol Policy. 2019; 2(1):59–83.18. Jung J. A study on Acts of the Republic of Korea regarding Technology Transfer and Commercialization from National Research and Development Project. Law Technol. 2014; 10(4):82–101.19. Ministry of Science and ICT. Rules for Management of Data from National Research and Development (Notice 2020-102). Sejong, Korea: Ministry of Science and ICT;2020.20. Yoon CM. Introduction and development direction of the national R&D data management system: focusing on the “Data Management Plan (DMP)” system. Sci Technol Law. 2020; 11(2):197–222.
Article21. National Institutes of Health. Supplemental information to the NIH policy for data management and sharing: protecting privacy when sharing human research participant data [Internet]. Bethesda (MD): National Institutes of Health;2020. [cited at 2023 Jul 31]. Available from: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-22-213.html.22. Lee WB. Adoption of Pseudonymization by Korea’s Personal Information Protection Act & Its Implications on Genomic Research. Ewha Law J. 2020; 25(1):191–224. https://doi.org/10.32632/elj.2020.25.1.191.
Article23. Molnar-Gabor F, Sellner J, Pagil S, Slokenberga S, Tzortzatou-Nanopoulou O, Nystrom K. Harmonization after the GDPR? Divergences in the rules for genetic and health data sharing in four member states and ways to overcome them by EU measures: insights from Germany, Greece, Latvia and Sweden. Semin Cancer Biol. 2022; 84:271–83. https://doi.org/10.1016/j.semcancer.2021.12.001.
Article24. Oh BC. EU Data Act Draft and Its Implication. Korean Forum Int Trade Bus Law. 2022; 31(1):487–516. https://doi.org/10.23068/KJITBL.2022.7.31.1.487.
Article25. Burk DL. Intellectual property in the context of e-Science. J Comput Mediat Commun. 2007; 12(2):600–17. https://doi.org/10.31235/osf.io/8agp9.
Article26. Dutfield G. Intellectual property rights and the life science industries: a twentieth century history. 1st ed. London, UK: Routledge;2003. https://doi.org/10.4324/9781315252131.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Health Promotion at Work: A Comparison of Policy and Practice Across Europe
- Work-Related Stress Risk Assessment in Italy: A Methodological Proposal Adapted to Regulatory Guidelines
- The Role of Academic Journal in Health Policy
- Physical activity recommendations and guidelines based on a new paradigm
- A Proposal for Health Insurance Policy to the New Administration