Intest Res.
2023 Jul;21(3):392-405. 10.5217/ir.2022.00094.
Prevalence of hepatitis B virus and hepatitis C virus infection in patients with inflammatory bowel disease: a systematic review and meta-analysis
- Affiliations
-
- 1Department of Gastroenterology, Nizam’s Institute of Medical Sciences, Hyderabad, India
- 2Department of Gastroenterology, PACE Hospital, Hyderabad, India
- 3Institute of Gastrosciences and Liver, Apollo Multispecialty Hospital, Kolkata, India
- 4Department of Gastroenterology, Fortis Hospital, Bengaluru, India
- 5Department of Gastroenterology, King Edward Memorial Hospital and Seth Gordhandas Sunderdas Medical College, Mumbai, India
- 6Department of Digestive Disease and Clinical Nutrition, Tata Memorial Hospital, Mumbai, India
- KMID: 2544762
- DOI: http://doi.org/10.5217/ir.2022.00094
Abstract
- Background/Aims
The data on the prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) infection in patients with inflammatory bowel disease (IBD) are conflicting. The present systematic review was thus conducted to study the prevalence of HBV and HCV markers in patients with IBD.
Methods
A comprehensive literature search of 3 databases was conducted from 2000 to April 2022 for studies evaluating the prevalence of HBV or HCV in patients with IBD. Pooled prevalence rates across studies were expressed with summative statistics.
Results
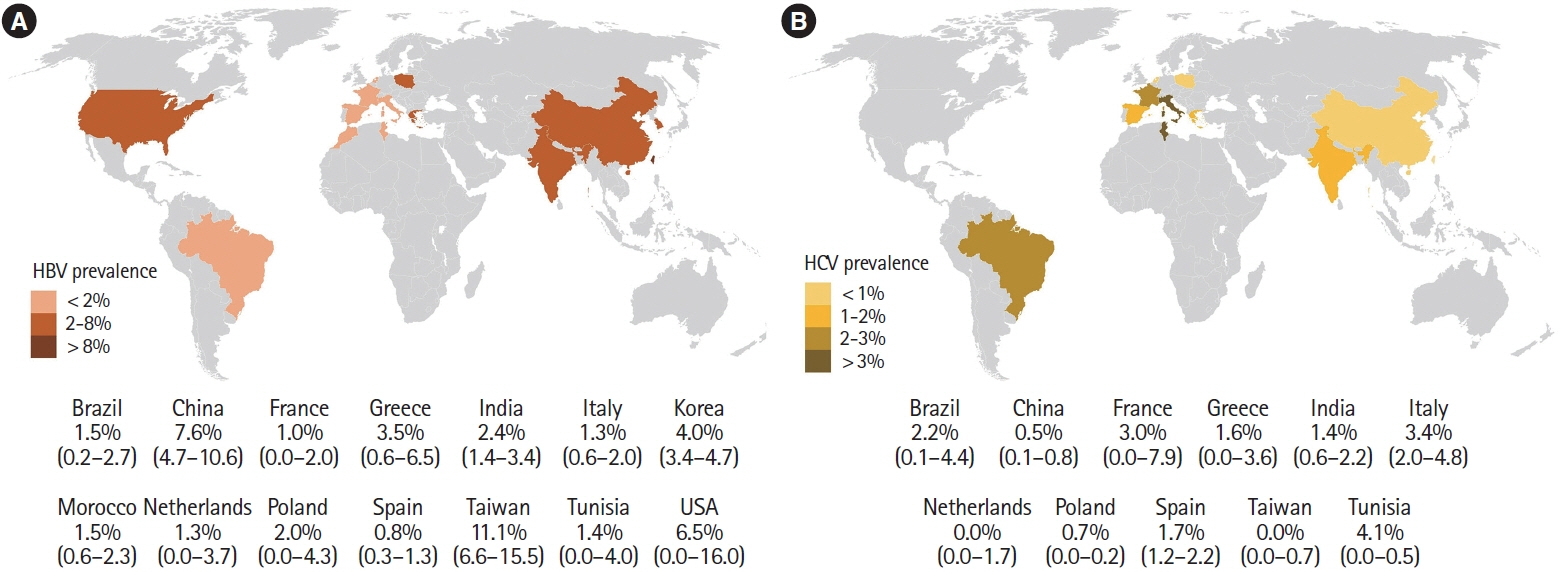
A total of 34 studies were included in the final analysis. The pooled prevalence of hepatitis B surface antigen (HBsAg) and hepatitis B core antibodies were 3.3% and 14.2%, respectively. In HBsAg positive IBD patients, hepatitis B e antigen positivity and detectable HBV DNA were seen in 15.3% and 61.0% of patients, respectively. Only 35.6% of the IBD patients had effective HBV vaccination. The pooled prevalence of anti-HCV and detectable HCV RNA were 1.8% and 0.8%, respectively. The pooled prevalence of markers of HBV infection was higher in Asian studies, while the prevalence of markers of HCV infection was higher in European studies. The prevalence of viral hepatitis markers was similar between IBD patients and the general population and that between ulcerative colitis and Crohn’s disease.
Conclusions
The prevalence of markers of viral hepatitis remains same as the general population with significant regional variations, although the quality of evidence remains low due to publication bias. Only a small proportion of IBD patients had an effective HBV vaccination, requiring improvement in screening and vaccination practices.
Figure
Cited by 1 articles
-
Infectious complications in patients with inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 8th Asian Organization for Crohn’s and Colitis meeting
Yu Kyung Jun, Seong-Joon Koh, Dae Seong Myung, Sang Hyoung Park, Choon Jin Ooi, Ajit Sood, Jong Pil Im
Intest Res. 2023;21(3):353-362. doi: 10.5217/ir.2023.00013.
Reference
-
1. Weimers P, Munkholm P. The natural history of IBD: lessons learned. Curr Treat Options Gastroenterol. 2018; 16:101–111.
Article2. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the AsiaPacific Crohn’s and Colitis Epidemiology Study. Gastroenterology. 2013; 145:158–165.
Article3. Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012; 27:1266–1280.
Article4. Gisbert JP, Chaparro M, Esteve M. Review article: prevention and management of hepatitis B and C infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011; 33:619–633.
Article5. Hou JK, Velayos F, Terrault N, Mahadevan U. Viral hepatitis and inflammatory bowel disease. Inflamm Bowel Dis. 2010; 16:925–932.
Article6. Reddy KR, Beavers KL, Hammond SP, Lim JK; Falck-Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015; 148:215–219.
Article7. Surveillance of viral hepatitis in Hong Kong-2012 update report [Internet]. c2014 [cited 2022 Aug 23]. https://www.hepatitis.gov.hk/english/health_professionals/files/hepsurv12.pdf.8. Principi M, Iannone A, Losurdo G, et al. Nonalcoholic fatty liver disease in inflammatory bowel disease: prevalence and risk factors. Inflamm Bowel Dis. 2018; 24:1589–1596.
Article9. Meijer B, van Everdingen CK, Ramsoekh D, et al. Transient elastography to assess liver stiffness in patients with inflammatory bowel disease. Dig Liver Dis. 2018; 50:48–53.
Article10. Rahier JF, Magro F, Abreu C, et al. Second European evidencebased consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014; 8:443–468.
Article11. Sansone S, Guarino M, Castiglione F, et al. Hepatitis B and C virus reactivation in immunosuppressed patients with inflammatory bowel disease. World J Gastroenterol. 2014; 20:3516–3524.
Article12. Kucharzik T, Ellul P, Greuter T, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. 2021; 15:879–913.
Article13. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis Of Observational Studies in Epidemiology: a proposal for reporting. JAMA. 2000; 283:2008–2012.
Article14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71.15. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020; 18:2127–2133.16. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188.
Article17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.
Article18. Longo F, Hebuterne X, Tran A, et al. Prevalence of hepatitis C in patients with chronic inflammatory bowel disease in the region of nice and evaluation of risk factors. Gastroenterol Clin Biol. 2000; 24:77–81.19. Biancone L, Pavia M, Del Vecchio Blanco G, et al. Hepatitis B and C virus infection in Crohn’s disease. Inflamm Bowel Dis. 2001; 7:287–294.
Article20. Esteve M, Saro C, González-Huix F, Suarez F, Forné M, Viver JM. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut. 2004; 53:1363–1365.
Article21. Tolentino YF, Fogaca HS, Zaltman C, Ximenes LL, Coelho HS. Hepatitis B virus prevalence and transmission risk factors in inflammatory bowel disease patients at Clementino Fraga Filho University Hospital. World J Gastroenterol. 2008; 14:3201–3206.
Article22. Agmon-Levin N, Ram M, Barzilai O, et al. Prevalence of hepatitis C serum antibody in autoimmune diseases. J Autoimmun. 2009; 32:261–266.
Article23. Lidar M, Langevitz P, Barzilai O, et al. Infectious serologies and autoantibodies in inflammatory bowel disease: insinuations at a true pathogenic role. Ann N Y Acad Sci. 2009; 1173:640–648.24. Loras C, Saro C, Gonzalez-Huix F, et al. Prevalence and factors related to hepatitis B and C in inflammatory bowel disease patients in Spain: a nationwide, multicenter study. Am J Gastroenterol. 2009; 104:57–63.
Article25. Chevaux JB, Nani A, Oussalah A, et al. Prevalence of hepatitis B and C and risk factors for nonvaccination in inflammatory bowel disease patients in Northeast France. Inflamm Bowel Dis. 2010; 16:916–924.
Article26. Katsanos KH, Tsianos VE, Zois CD, et al. Inflammatory bowel disease and hepatitis B and C in Western Balkans: a referral centre study and review of the literature. J Crohns Colitis. 2010; 4:450–465.
Article27. Morisco F, Castiglione F, Rispo A, et al. Effect of immunosuppressive therapy on patients with inflammatory bowel diseases and hepatitis B or C virus infection. J Viral Hepat. 2013; 20:200–208.
Article28. Park SH, Yang SK, Lim YS, et al. Clinical courses of chronic hepatitis B virus infection and inflammatory bowel disease in patients with both diseases. Inflamm Bowel Dis. 2012; 18:2004–2010.
Article29. Vaughn BP, Doherty GA, Gautam S, Moss AC, Cheifetz AS. Screening for tuberculosis and hepatitis B prior to the initiation of anti-tumor necrosis therapy. Inflamm Bowel Dis. 2012; 18:1057–1063.
Article30. Kim ES, Cho KB, Park KS, et al. Prevalence of hepatitis-B viral markers in patients with inflammatory bowel disease in a hepatitis-B-endemic area: inadequate protective antibody levels in young patients. J Clin Gastroenterol. 2014; 48:553–558.
Article31. Papa A, Felice C, Marzo M, et al. Prevalence and natural history of hepatitis B and C infections in a large population of IBD patients treated with anti-tumor necrosis factor-α agents. J Crohns Colitis. 2013; 7:113–119.
Article32. Ben Musa R, Gampa A, Basu S, et al. Hepatitis B vaccination in patients with inflammatory bowel disease. World J Gastroenterol. 2014; 20:15358–15366.
Article33. Huang ML, Xu XT, Shen J, Qiao YQ, Dai ZH, Ran ZH. Prevalence and factors related to hepatitis B and C infection in inflammatory bowel disease patients in China: a retrospective study. J Crohns Colitis. 2014; 8:282–287.
Article34. Loras C, Gisbert JP, Saro MC, et al. Impact of surveillance of hepatitis B and hepatitis C in patients with inflammatory bowel disease under anti-TNF therapies: multicenter prospective observational study (REPENTINA 3). J Crohns Colitis. 2014; 8:1529–1538.
Article35. Sui M, Wu R, Hu X, et al. Low prevalence of hepatitis B virus infection in patients with autoimmune diseases in a Chinese patient population. J Viral Hepat. 2014; 21:925–929.
Article36. van der Have M, Belderbos TD, Fidder HH, et al. Screening prior to biological therapy in Crohn’s disease: adherence to guidelines and prevalence of infections: results from a multicentre retrospective study. Dig Liver Dis. 2014; 46:881–886.
Article37. He Y, Xu P, Chen Y, et al. Prevalence and influences of hepatitis B virus infection on inflammatory bowel disease: a retrospective study in Southern China. Int J Clin Exp Med. 2015; 8:8078–8085.38. Chan HC, Wong VW, Wong GL, Tang W, Wu JC, Ng SC. Prevalence of hepatitis B and clinical outcomes in inflammatory bowel disease patients in a viral-endemic region. BMC Gastroenterol. 2016; 16:100.
Article39. Waszczuk E, Waszczuk KM, Mulak A, Paradowski L. Inadequate seroprotection against hepatitis B virus and one detected case of hepatitis C virus infection among patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2016; 28:628–632.
Article40. Ardesia M, Costantino G, Mondello P, Alibrandi A, Fries W. Serology of viral infections and tuberculosis screening in an IBD population referred to a tertiary centre of Southern Italy. Gastroenterol Res Pract. 2017; 2017:4139656.
Article41. Chen D, Luo S, Ben Q, Lu L, Wan X, Wu J. Prevalence of hepatitis B and C and factors for infection and nonimmune in inflammatory bowel disease patients in China. Eur J Gastroenterol Hepatol. 2017; 29:509–515.
Article42. Harsh P, Gupta V, Kedia S, et al. Prevalence of hepatitis B, hepatitis C and human immunodeficiency viral infections in patients with inflammatory bowel disease in north India. Intest Res. 2017; 15:97–102.
Article43. Abid H, Meyiz H, Laalaj O, et al. Viral hepatitis B during chronic inflammatory bowel diseases at Fez University Hospital: prevalence and risk factors. Open J Gastroenterol. 2018; 8:17–26.
Article44. Shah R, Ho EY, Kramer JR, et al. Hepatitis B virus screening and reactivation in a national VA cohort of patients with inflammatory bowel disease treated with tumor necrosis factor antagonists. Dig Dis Sci. 2018; 63:1551–1557.
Article45. Yeo SJ, Lee HS, Jang BI, et al. Nonimmunity against hepatitis B virus infection in patients newly diagnosed with inflammatory bowel disease. Intest Res. 2018; 16:400–408.
Article46. Chou JW, Lai HC, Chang CH, Cheng KS, Feng CL, Chen TW. Epidemiology and clinical outcomes of inflammatory bowel disease: a hospital-based study in central Taiwan. Gastroenterol Res Pract. 2019; 2019:4175923.
Article47. Fousekis FS, Katsanos KH, Theopistos VI, et al. Hepatobiliary and pancreatic manifestations in inflammatory bowel diseases: a referral center study. BMC Gastroenterol. 2019; 19:48.
Article48. Silva J, Brito BS, Silva IN, et al. Frequency of hepatobiliary manifestations and concomitant liver disease in inflammatory bowel disease patients. Biomed Res Int. 2019; 2019:7604939.
Article49. Losurdo G, Iannone A, Contaldo A, et al. Chronic viral hepatitis in a cohort of inflammatory bowel disease patients from Southern Italy: a case-control study. Pathogens. 2020; 9:870.
Article50. Sabbah M, Yacoub H, Bellil N, et al. Hepatitis B and C viral infections screening in a Tunisian IBD population under immunosuppressive therapies. Rev Gastroenterol Peru. 2020; 40:246–251.
Article51. Patil AP, Simon EG, Dutta AK, Joseph AJ, Kurien RT, Chowdhury SD. Prevalence of serological markers of hepatitis B in inflammatory bowel disease: experience from a tertiary care centre in South India. Trop Doct. 2021; 51:326–331.
Article52. Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018; 3:383–403.53. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020; 5:17–30.54. Childs L, Roesel S, Tohme RA. Status and progress of hepatitis B control through vaccination in the South-East Asia region, 1992-2015. Vaccine. 2018; 36:6–14.
Article55. Loras C, Gisbert JP, Mínguez M, et al. Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut. 2010; 59:1340–1346.
Article56. Lee JM, Wei SC, Lee KM, et al. Clinical course of hepatitis B viral infection in patients undergoing anti-tumor necrosis factor α therapy for inflammatory bowel disease. Gut Liver. 2022; 16:396–403.
Article57. Lamb CA, Kennedy NA, Raine T, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019; 68(Suppl 3):s1–s106.58. Kochhar GS, Mohan BP, Khan SR, et al. Hepatitis-B vaccine response in inflammatory bowel disease patients: a systematic review and meta-analysis. Inflamm Bowel Dis. 2021; 27:1610–1619.59. Gupta A, Macrae FA, Gibson PR. Vaccination and screening for infections in patients with inflammatory bowel disease: a survey of Australian gastroenterologists. Intern Med J. 2011; 41:462–467.60. Poupardin C, Nahon S, Pariente A, Cadranel JF, Renou C; ANGH. Hepatitis B reactivation in patients with inflammatory bowel disease: a prospective survey on screening and prevention practices at general hospitals in France. Inflamm Bowel Dis. 2011; 17:669–670.61. Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022; 7:396–415.62. Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016; 22:7824–7840.63. European Centre for Disease Prevention and Control. Prevention of hepatitis B and C in the EU/EEA and the UK [Internet]. c2020 [cited 2022 Aug 23]. https://www.ecdc.europa.eu/sites/default/files/documents/hepatitis-B-and-C-prevention_1.pdf.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevention of Viral Hepatitis and Vaccination
- Pre-S Defective Hepatitis B Virus in Patients with Acute and chronic Hepatitis B Virus Infection
- Prevalence and Clinical Implications of Occult Hepatitis B Virus Infection
- A Study of Hepatitis B Virus Infection in Patients with Hand Eczema
- Occult Hepatitis B Virus Infection in Patients with Chronic Hepatitis C: Innocent Bystander or Not?