Clin Endosc.
2023 Jul;56(4):510-520. 10.5946/ce.2022.142.
Diagnostic value of homogenous delayed enhancement in contrast-enhanced computed tomography images and endoscopic ultrasound-guided tissue acquisition for patients with focal autoimmune pancreatitis
- Affiliations
-
- 1Department of Gastroenterology, Sendai City Medical Center, Sendai, Japan
- 2Department of Gastroenterological Surgery, Sendai City Medical Center, Sendai, Japan
- 3Department of Pathology, Sendai City Medical Center, Sendai, Japan
- KMID: 2544574
- DOI: http://doi.org/10.5946/ce.2022.142
Abstract
- Background/Aims
We aimed to investigate (1) promising clinical findings for the recognition of focal type autoimmune pancreatitis (FAIP) and (2) the impact of endoscopic ultrasound (EUS)-guided tissue acquisition (EUS-TA) on the diagnosis of FAIP.
Methods
Twenty-three patients with FAIP were involved in this study, and 44 patients with resected pancreatic ductal adenocarcinoma (PDAC) were included in the control group.
Results
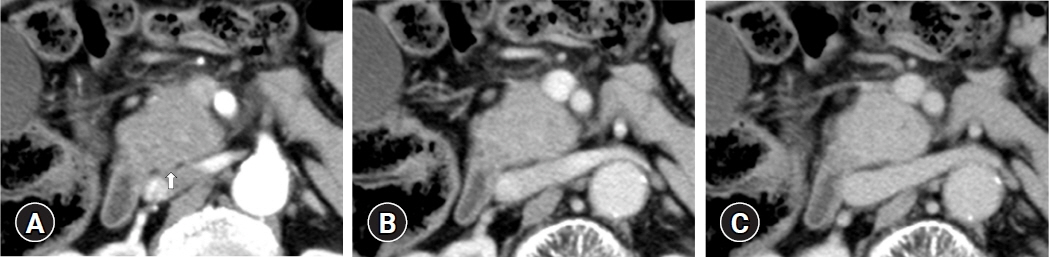
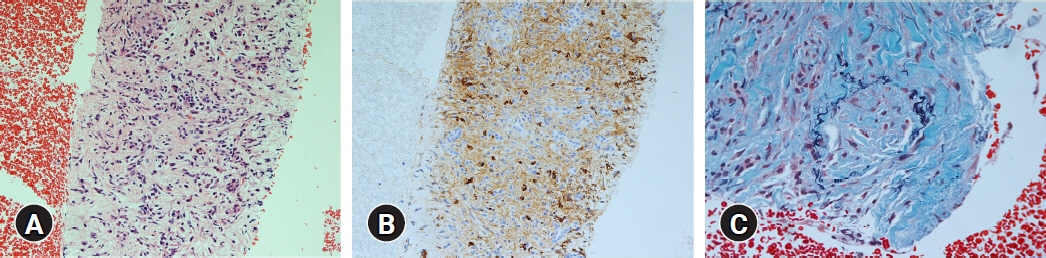
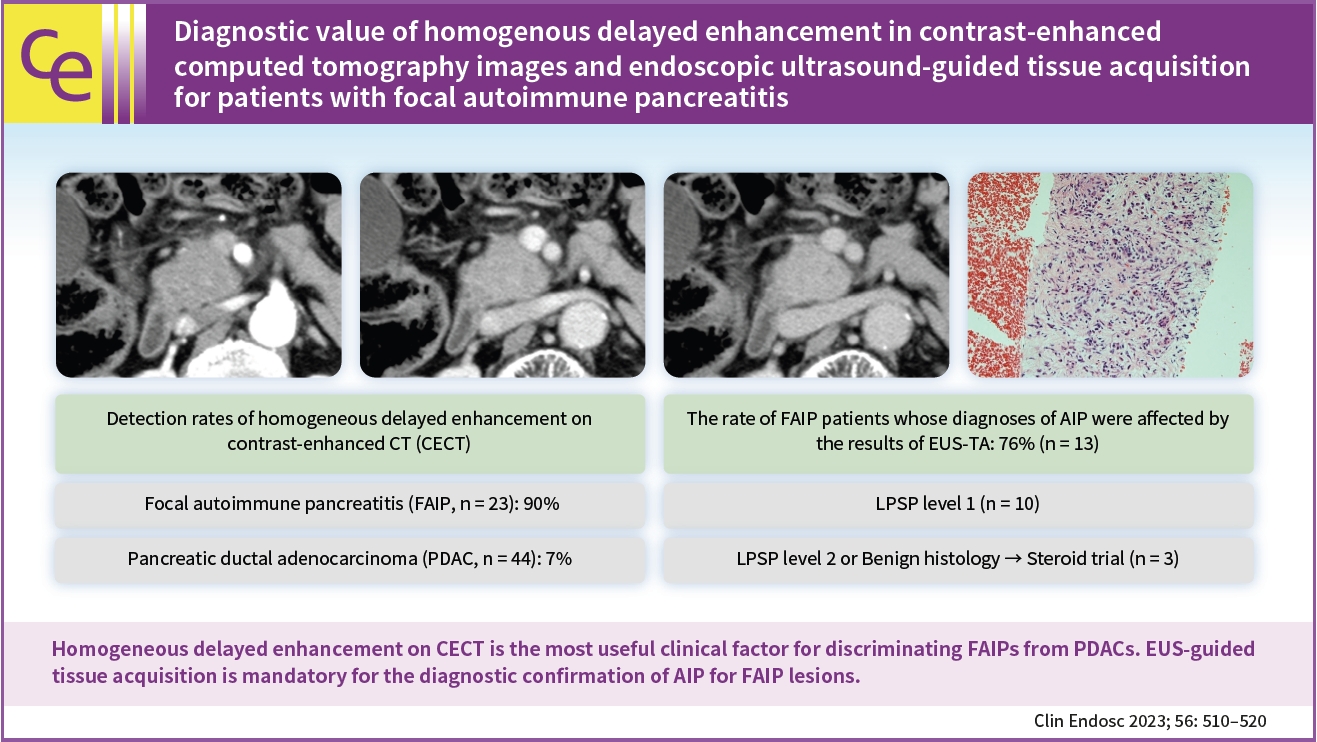
(1) Multivariate analysis revealed that homogeneous delayed enhancement on contrast-enhanced computed tomography was a significant factor indicative of FAIP compared to PDAC (90% vs. 7%, p=0.015). (2) For 13 of 17 FAIP patients (76.5%) who underwent EUS-TA, EUS-TA aided the diagnostic confirmation of AIPs, and only one patient (5.9%) was found to have AIP after surgery. On the other hand, of the six patients who did not undergo EUS-TA, three (50.0%) underwent surgery for pancreatic lesions.
Conclusions
Homogeneous delayed enhancement on contrast-enhanced computed tomography was the most useful clinical factor for discriminating FAIPs from PDACs. EUS-TA is mandatory for diagnostic confirmation of FAIP lesions and can contribute to a reduction in the rate of unnecessary surgery for patients with FAIP.
Keyword
Figure
Reference
-
1. Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003; 38:982–984.2. Kamisawa T, Funata N, Hayashi Y, et al. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003; 52:683–687.3. Okazaki K, Kawa S, Kamisawa T, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006; 41:626–631.4. Kawaguchi K, Koike M, Tsuruta K, et al. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991; 22:387–395.5. Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011; 40:352–358.6. Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut. 2013; 62:1771–1776.7. Chang MC, Liang PC, Jan S, et al. Increase diagnostic accuracy in differentiating focal type autoimmune pancreatitis from pancreatic cancer with combined serum IgG4 and CA19-9 levels. Pancreatology. 2014; 14:366–372.8. Pak LM, Schattner MA, Balachandran V, et al. The clinical utility of immunoglobulin G4 in the evaluation of autoimmune pancreatitis and pancreatic adenocarcinoma. HPB (Oxford). 2018; 20:182–187.9. Furuhashi N, Suzuki K, Sakurai Y, et al. Differentiation of focal-type autoimmune pancreatitis from pancreatic carcinoma: assessment by multiphase contrast-enhanced CT. Eur Radiol. 2015; 25:1366–1374.10. He C, Rong D, Hu W, et al. A feasible CT feature to differentiate focal-type autoimmune pancreatitis from pancreatic ductal adenocarcinoma. Cancer Med. 2019; 8:6250–6257.11. Zaheer A, Singh VK, Akshintala VS, et al. Differentiating autoimmune pancreatitis from pancreatic adenocarcinoma using dual-phase computed tomography. J Comput Assist Tomogr. 2014; 38:146–152.12. Takahashi N, Fletcher JG, Hough DM, et al. Autoimmune pancreatitis: differentiation from pancreatic carcinoma and normal pancreas on the basis of enhancement characteristics at dual-phase CT. AJR Am J Roentgenol. 2009; 193:479–484.13. Lee-Felker SA, Felker ER, Kadell B, et al. Use of MDCT to differentiate autoimmune pancreatitis from ductal adenocarcinoma and interstitial pancreatitis. AJR Am J Roentgenol. 2015; 205:2–9.14. Sugumar A, Levy MJ, Kamisawa T, et al. Endoscopic retrograde pancreatography criteria to diagnose autoimmune pancreatitis: an international multicentre study. Gut. 2011; 60:666–670.15. Kanno A, Masamune A, Fujishima F, et al. Diagnosis of autoimmune pancreatitis by EUS-guided FNA using a 22-gauge needle: a prospective multicenter study. Gastrointest Endosc. 2016; 84:797–804.16. Morishima T, Kawashima H, Ohno E, et al. Prospective multicenter study on the usefulness of EUS-guided FNA biopsy for the diagnosis of autoimmune pancreatitis. Gastrointest Endosc. 2016; 84:241–248.17. Iwashita T, Yasuda I, Doi S, et al. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2012; 10:316–322.18. Kurita A, Yasukawa S, Zen Y, et al. Comparison of a 22-gauge Franseen-tip needle with a 20-gauge forward-bevel needle for the diagnosis of type 1 autoimmune pancreatitis: a prospective, randomized, controlled, multicenter study (COMPAS study). Gastrointest Endosc. 2020; 91:373–381.19. Ishikawa T, Kawashima H, Ohno E, et al. Usefulness of endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of autoimmune pancreatitis using a 22-gauge Franseen needle: a prospective multicenter study. Endoscopy. 2020; 52:978–985.20. Koshita S, Ito K, Fujita N, et al. Localized autoimmune pancreatitis, 9 mm in size, without strictures of the main pancreatic duct. Gastrointest Endosc. 2012; 75:920–922.21. Cao Z, Tian R, Zhang T, et al. Localized autoimmune pancreatitis: report of a case clinically mimicking pancreatic cancer and a literature review. Medicine (Baltimore). 2015; 94:e1656.22. Noda Y, Fujita N, Kobayashi G, et al. Diagnostic efficacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound-guided fine-needle aspiration. J Gastroenterol. 2010; 45:868–875.23. Sumimoto K, Uchida K, Mitsuyama T, et al. A proposal of a diagnostic algorithm with validation of international consensus diagnostic criteria for autoimmune pancreatitis in a Japanese cohort. Pancreatology. 2013; 13:230–237.24. Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012; 75:319–331.25. Khan MA, Grimm IS, Ali B, et al. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017; 5:E363–E375.26. Chen G, Liu S, Zhao Y, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: a meta-analysis. Pancreatology. 2013; 13:298–304.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Contrast-enhanced endoscopic ultrasound for pancreatobiliary disease
- Contrast Harmonic Endoscopic Ultrasound in Pancreatic Diseases
- Endoscopic ultrasound-guided tissue acquisition: Needle types, technical issues, and sample handling
- Fine-Needle Biopsy: Should This Be the First Choice in Endoscopic Ultrasound-Guided Tissue Acquisition?
- Diagnostic Endoscopic Ultrasound: Technique, Current Status and Future Directions