Cancer Res Treat.
2023 Jul;55(3):939-947. 10.4143/crt.2022.1645.
Intraindividual Comparison of MRIs with Extracellular and Hepatobiliary Contrast Agents for the Noninvasive Diagnosis of Hepatocellular Carcinoma Using the Korean Liver Cancer Association–National Cancer Center 2022 Criteria
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2544174
- DOI: http://doi.org/10.4143/crt.2022.1645
Abstract
- Purpose
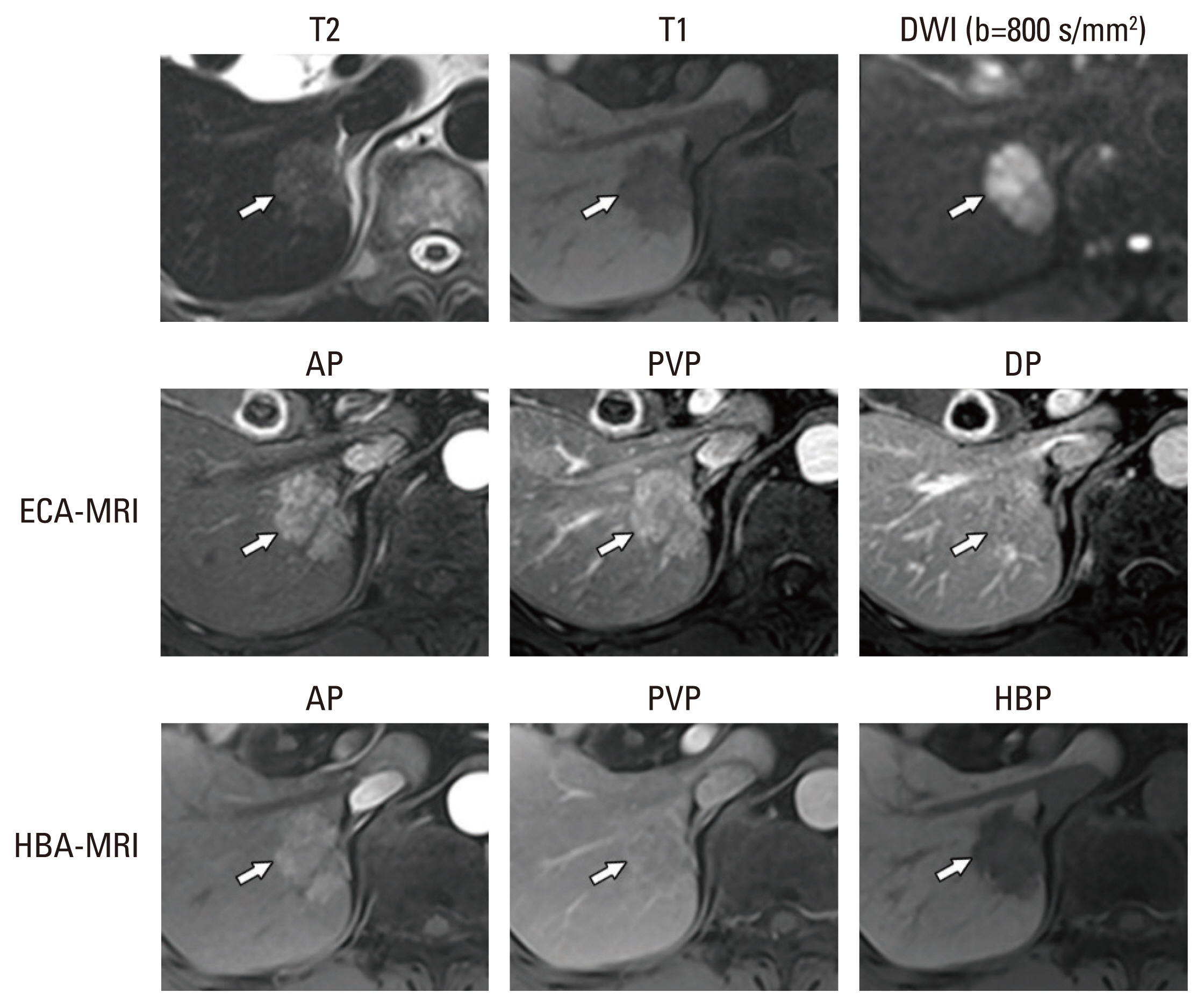
The aim of the present study was to evaluate the per-lesion sensitivity and specificity of the Korean Liver Cancer Association–National Cancer Center (KLCA-NCC) 2022 criteria for the noninvasive diagnosis of hepatocellular carcinoma (HCC), with intraindividual comparison of the diagnostic performance of magnetic resonance imaging with extracellular agents (ECA-MRI) and hepatobiliary agents (HBA-MRI).
Materials and Methods
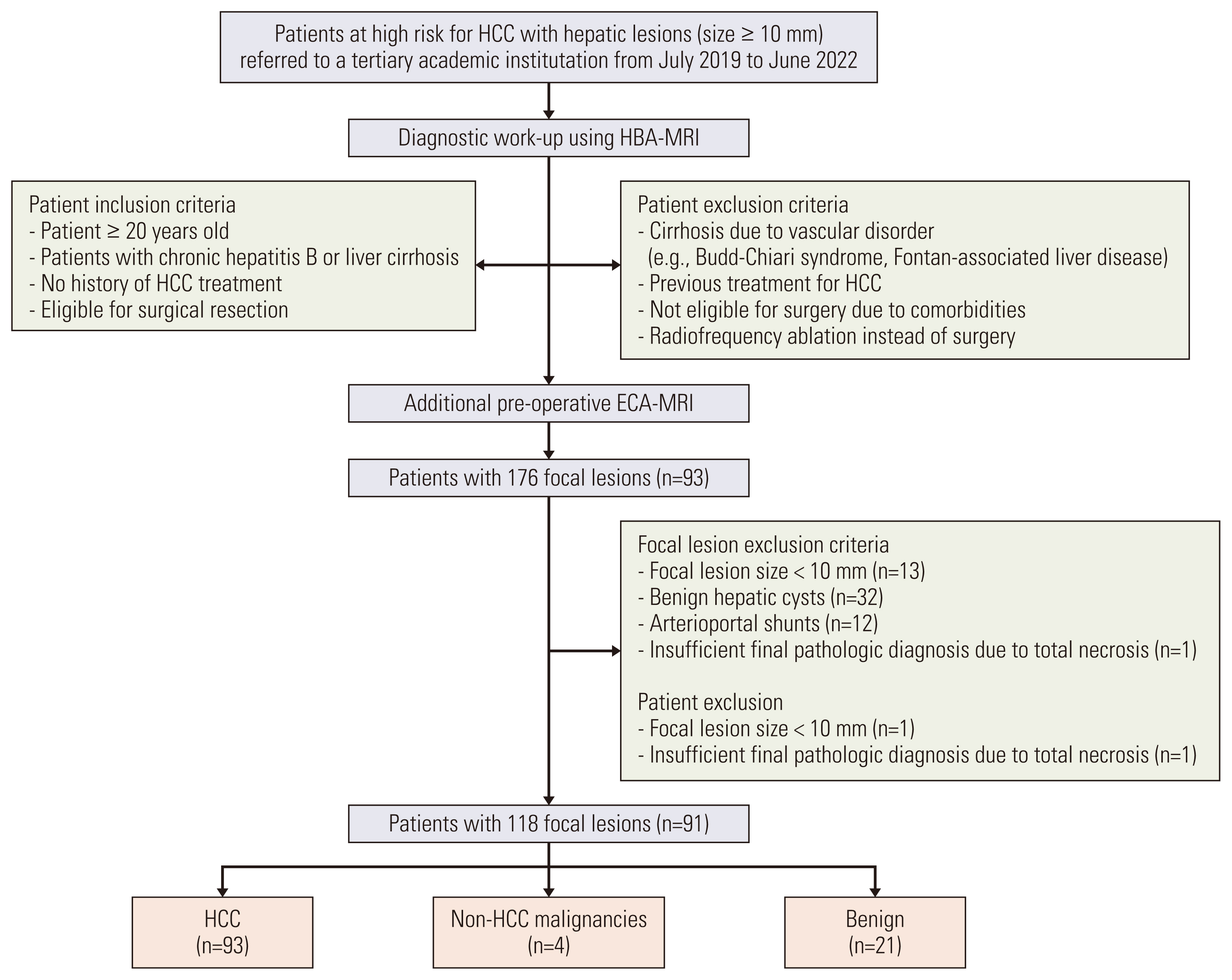
Patients at high risk for HCC who were referred to a tertiary academic institution for hepatic lesions with size ≥ 10 mm between July 2019 and June 2022 were enrolled. A total of 91 patients (mean age, 58.1 years; 76 men and 15 women) with 118 lesions who underwent both ECA-MRI and HBA-MRI were eligible for final analysis. The per-lesion sensitivities and specificities of the KLCA-NCC 2022 criteria using ECA-MRI and HBA-MRI were compared using McNemar’s test.
Results
The 118 lesions were 93 HCCs, 4 non-HCC malignancies, and 21 benign lesions. On HBA-MRI, the “definite” HCC category showed significantly higher sensitivity than ECA-MRI (78.5% vs. 58.1%, p < 0.001), with identical specificity (92.0% vs. 92.0%, p > 0.999). For “probable” or “definite” HCC categories, there were no differences in the sensitivity (84.9% vs. 84.9%, p > 0.999) and specificity (84.0% vs. 84.0%, p > 0.999) between ECA-MRI and HBA-MRI.
Conclusion
The “definite” HCC category of the KLCA-NCC 2022 criteria showed higher sensitivity in diagnosing HCC on HBA-MRI compared with ECA-MRI, without compromising specificity. There were no significant differences in the sensitivity and specificity of “probable” or “definite” HCC categories according to ECA-MRI and HBA-MRI.
Figure
Reference
-
References
1. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Clin Liver Dis. 2019; 13:1.
Article2. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019; 70:151–71.
Article3. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017; 11:317–70.
Article4. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.5. American College of Radiology. Committee on LI-RADS® (Liver). Liver Imaging Reporting and Data System (LI-RADS) version 2018 Core [Internet]. Reston, VA: American College of Radiology;2018. [cited 2022 Oct 1]. Available from: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf?la=en.6. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC). 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2019; 20:1042–113.7. Kim TH, Kim SY, Tang A, Lee JM. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol. 2019; 25:245–63.
Article8. Korean Liver Cancer Association (KLCA); National Cancer Center. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022; 28:583–705.9. Kim TK, Lee KH, Jang HJ, Haider MA, Jacks LM, Menezes RJ, et al. Analysis of gadobenate dimeglumine-enhanced MR findings for characterizing small (1–2-cm) hepatic nodules in patients at high risk for hepatocellular carcinoma. Radiology. 2011; 259:730–8.
Article10. Tang A, Bashir MR, Corwin MT, Cruite I, Dietrich CF, Do RK, et al. Evidence supporting LI-RADS major features for CT- and MR imaging-based diagnosis of hepatocellular carcinoma: a systematic review. Radiology. 2018; 286:29–48.11. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960; 20:37–46.
Article12. Byun J, Choi SH, Byun JH, Lee SJ, Kim SY, Won HJ, et al. Comparison of the diagnostic performance of imaging criteria for HCCs ≤ 3.0 cm on gadoxetate disodium-enhanced MRI. Hepatol Int. 2020; 14:534–43.13. Jeon SK, Lee JM, Joo I, Yoo J, Park JY. Comparison of guidelines for diagnosis of hepatocellular carcinoma using gadoxetic acid-enhanced MRI in transplantation candidates. Eur Radiol. 2020; 30:4762–71.
Article14. Lee S, Kim MJ. Validation of the Korean Liver Cancer Association-National Cancer Center 2018 criteria for the noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging. J Liver Cancer. 2020; 20:120–7.
Article15. Lee S, Kim SS, Chang DR, Kim H, Kim MJ. Comparison of LI-RADS 2018 and KLCA-NCC 2018 for noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging. Clin Mol Hepatol. 2020; 26:340–51.
Article16. Kim DH, Kim B, Youn SY, Kim H, Choi JI. Diagnostic performance of KLCA-NCC 2018 criteria for hepatocellular carcinoma using magnetic resonance imaging: a systematic review and meta-analysis. Diagnostics (Basel). 2021; 11:1763.
Article17. Hwang SH, Park MS, Park S, Lim JS, Kim SU, Park YN. Comparison of the current guidelines for diagnosing hepatocellular carcinoma using gadoxetic acid-enhanced magnetic resonance imaging. Eur Radiol. 2021; 31:4492–503.
Article18. Park SH, Shim YS, Kim B, Kim SY, Kim YS, Huh J, et al. Retrospective analysis of current guidelines for hepatocellular carcinoma diagnosis on gadoxetic acid-enhanced MRI in at-risk patients. Eur Radiol. 2021; 31:4751–63.
Article19. Lee SM, Lee JM, Ahn SJ, Kang HJ, Yang HK, Yoon JH. Diagnostic performance of 2018 KLCA-NCC practice guideline for hpatocellular carcinoma on gadoxetic acid-enhanced MRI in patients with chronic hepatitis B or cirrhosis: comparison with LI-RADS version 2018. Korean J Radiol. 2021; 22:1066–76.
Article20. Joo I, Lee JM, Lee DH, Jeon JH, Han JK, Choi BI. Noninvasive diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout? Eur Radiol. 2015; 25:2859–68.21. Choi MH, Choi JI, Lee YJ, Park MY, Rha SE, Lall C. MRI of small hepatocellular carcinoma: typical features are less frequent below a size cutoff of 1.5 cm. AJR Am J Roentgenol. 2017; 208:544–51.
Article22. Yoon J, Hwang JA, Lee S, Lee JE, Ha SY, Park YN. Clinicopathologic and MRI features of combined hepatocellular-cholangiocarcinoma in patients with or without cirrhosis. Liver Int. 2021; 41:1641–51.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Emerging Role of Hepatobiliary Magnetic Resonance Contrast Media and Contrast-Enhanced Ultrasound for Noninvasive Diagnosis of Hepatocellular Carcinoma: Emphasis on Recent Updates in Major Guidelines
- Sonazoid-enhanced ultrasonography for noninvasive imaging diagnosis of hepatocellular carcinoma: special emphasis on the 2022 KLCA-NCC guideline
- Diagnostic performance of the 2022 KLCA-NCC criteria for hepatocellular carcinoma on magnetic resonance imaging with extracellular contrast and hepatobiliary agents: comparison with the 2018 KLCA-NCC criteria
- Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update
- Validation of the Korean Liver Cancer Association-National Cancer Center 2018 Criteria for the Noninvasive Diagnosis of Hepatocellular Carcinoma Using Magnetic Resonance Imaging