J Korean Neurosurg Soc.
2023 Jul;66(4):465-475. 10.3340/jkns.2022.0166.
Leptomeningeal Metastasis in Gliomas : Clinical Characteristics and Risk Factors
- Affiliations
-
- 1Department of Neurosurgery, Myongji Hospital, Goyang, Korea
- 2Neuro-oncology Clinic, National Cancer Center, Goyang, Korea
- 3Department of Radiology, National Cancer Center, Goyang, Korea
- 4Department of Cancer Control, Graduate School of Cancer Science and Policy, Goyang, Korea
- KMID: 2543538
- DOI: http://doi.org/10.3340/jkns.2022.0166
Abstract
Objective
: Our objective is to analyze the occurrence, clinical course and risk factors for glioma patients with leptomeningeal metastasis (LM) according to different metastasis patterns and clinical variables.
Methods
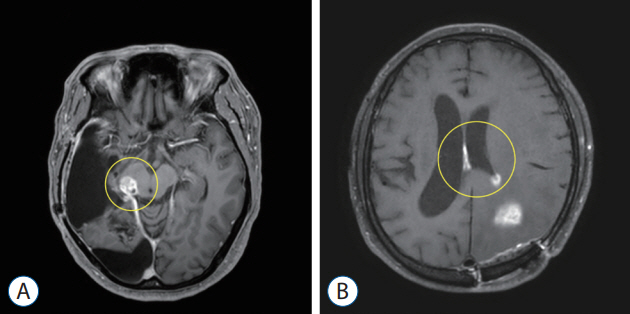
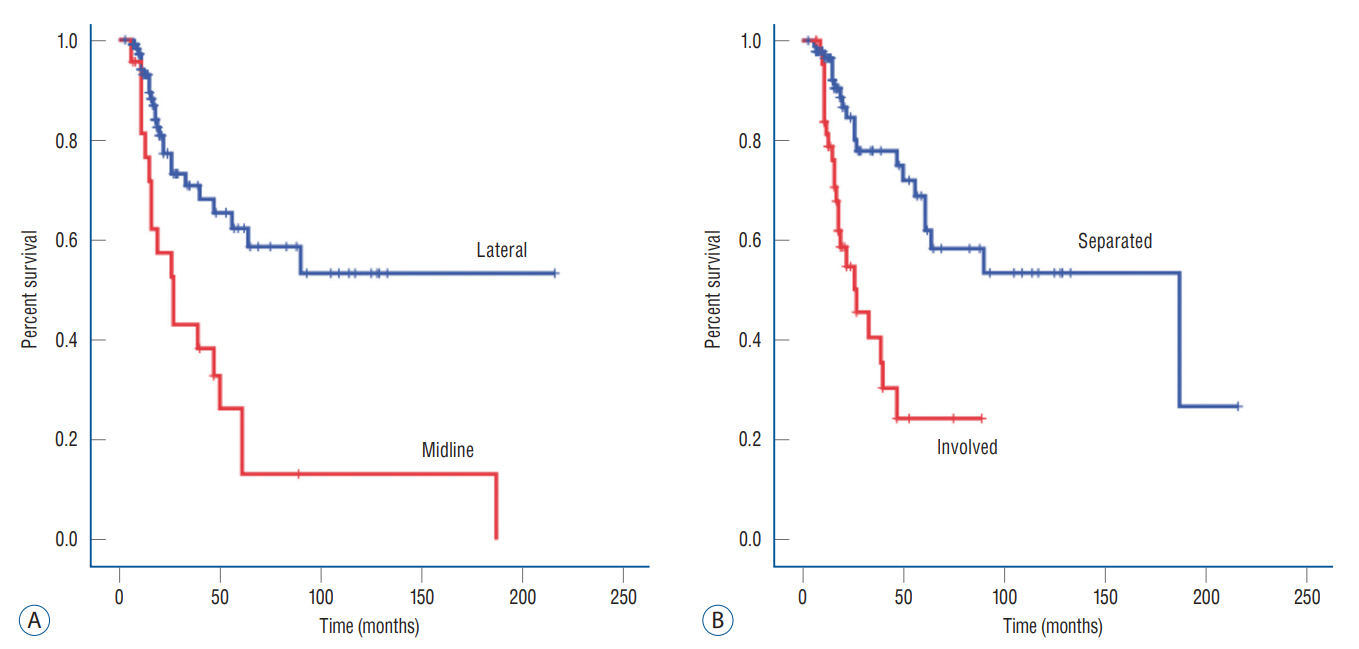
: We retrospectively reviewed data from 376 World Health Organization (WHO) grade II–IV adult glioma patients who were treated in the National Cancer Center from 2001 to 2020. Patients who underwent surgery at other institutions, those without initial images or those with pathologically unconfirmed cases were excluded. LM was diagnosed based on magnetic resonance imaging (MRI) findings or cerebrospinal fluid (CSF) cytology. The metastasis pattern was categorized as nodular or linear according to the enhancement pattern. Tumor proximity to the CSF space was classified as involved or separated, whereas location of the tumor was dichotomized as midline, for tumors residing in the thalamus, basal ganglia and brainstem, or lateral, for tumors residing in the cerebral and cerebellar hemispheres.
Results
: A total of 138 patients were enrolled in the study. A total of 44 patients (38%) were diagnosed with LM during a median follow-up of 9 months (range, 0–60). Among the clinical variables, tumor proximity to CSF space, the location of the tumor and the WHO grade were significant factors for LM development in univariate analysis. In multivariate analysis, the midline location of the tumor and WHO grade IV gliomas were the most significant factor for LM development. The hazard ratio was 2.624 for midline located gliomas (95% confidence interval [CI], 1.384–4.974; p=0.003) and 3.008 for WHO grade IV gliomas (95% CI, 1.379–6.561; p=0.006).
Conclusion
: Midline location and histological grading are an important factor for LM in glioma patients. The proximity to the CSF circulation pathway is also an important factor for WHO grade IV glioma LM. Patients carrying high risks should be followed up more thoroughly.
Figure
Reference
-
References
1. Ahn JH, Lee SH, Kim S, Joo J, Yoo H, Lee SH, et al. Risk for leptomeningeal seeding after resection for brain metastases: implication of tumor location with mode of resection. J Neurosurg. 116:984–993. 2012.
Article2. Andersen BM, Miranda C, Hatzoglou V, DeAngelis LM, Miller AM. Leptomeningeal metastases in glioma: the Memorial Sloan Kettering Cancer Center experience. Neurology. 92:e2483–e2491. 2019.3. Awad I, Bay JW, Rogers L. Leptomeningeal metastasis from supratentorial malignant gliomas. Neurosurgery. 19:247–251. 1986.
Article4. Bae JS, Yang SH, Yoon WS, Kang SG, Hong YK, Jeun SS. The clinical features of spinal leptomeningeal dissemination from malignant gliomas. J Korean Neurosurg Soc. 49:334–338. 2011.
Article5. Bae JW, Hong EK, Gwak HS. Response of leptomeningeal dissemination of anaplastic glioma to temozolomide: experience of two cases. Brain Tumor Res Treat. 5:99–104. 2017.
Article6. Bordignon KC, Neto MC, Ramina R, de Meneses MS, Zazula AD, de Almeida LG. Patterns of neuroaxis dissemination of gliomas: suggestion of a classification based on magnetic resonance imaging findings. Surg Neurol. 65:472–477. discussion 477. 2006.
Article7. Brower JV, Saha S, Rosenberg SA, Hullett CR, Ian Robins H. Management of leptomeningeal metastases: prognostic factors and associated outcomes. J Clin Neurosci. 27:130–137. 2016.
Article8. Bush NA, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev. 40:1–14. 2017.
Article9. Cinalli G, Imperato A, Mirone G, Di Martino G, Nicosia G, Ruggiero C, et al. Initial experience with endoscopic ultrasonic aspirator in purely neuroendoscopic removal of intraventricular tumors. J Neurosurg Pediatr. 19:325–332. 2017.
Article10. Dardis C, Milton K, Ashby L, Shapiro W. Leptomeningeal metastases in high-grade adult glioma: development, diagnosis, management, and outcomes in a series of 34 patients. Front Neurol. 5:220. 2014.
Article11. Eade OE, Urich H. Metastasising gliomas in young subjects. J Pathol. 103:245–256. 1971.
Article12. Freilich RJ, Krol G, Deangelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 38:51–57. 1995.
Article13. Hansen N, Wittig A, Hense J, Kastrup O, Gizewski ER, Van de Nes JA. Long survival of primary diffuse leptomeningeal gliomatosis following radiotherapy and temozolomide: case report and literature review. Eur J Med Res. 16:415–419. 2011.
Article14. Jung JM, Kim S, Joo J, Shin KH, Gwak HS, Lee SH. Incidence and risk factors for leptomeningeal carcinomatosis in breast cancer patients with parenchymal brain metastases. J Korean Neurosurg Soc. 52:193–199. 2012.
Article15. Louis DN, Giannini C, Capper D, Paulus W, Figarella-Branger D, Lopes MB, et al. cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol. 135:639–642. 2018.
Article16. Mandel JJ, Yust-Katz S, Cachia D, Wu J, Liu D, de Groot JF, et al. Leptomeningeal dissemination in glioblastoma; an inspection of risk factors, treatment, and outcomes at a single institution. J Neurooncol. 120:597–605. 2014.
Article17. Michotte A, Chaskis C, Sadones J, Veld PI, Neyns B. Primary leptomeningeal anaplastic oligodendroglioma with a 1p36-19q13 deletion: report of a unique case successfully treated with temozolomide. J Neurol Sci. 287:267–270. 2009.
Article18. Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky performance status scale. An examination of its reliability and validity in a research setting. Cancer. 53:2002–2007. 1984.
Article19. Nandipati S, Demopoulos A. Leptomeningeal dissemination of anaplastic glioma: prolonged survival in two patients treated with temozolomide. J Neurooncol. 105:663–665. 2011.
Article20. Onda K, Tanaka R, Takahashi H, Takeda N, Ikuta F. Cerebral glioblastoma with cerebrospinal fluid dissemination: a clinicopathological study of 14 cases examined by complete autopsy. Neurosurgery. 25:533–540. 1989.
Article21. Parsa AT, Wachhorst S, Lamborn KR, Prados MD, McDermott MW, Berger MS, et al. Prognostic significance of intracranial dissemination of glioblastoma multiforme in adults. J Neurosurg. 102:622–628. 2005.
Article22. Preston JK, Masciopinto J, Salamat MS, Badie B. Tumour cell dispersion by the ultrasonic aspirator during brain tumour resection. Br J Neurosurg. 13:486–489. 1999.
Article23. Qiu T, Chanchotisatien A, Qin Z, Wu J, Du Z, Zhang X, et al. Imaging characteristics of adult H3 K27M-mutant gliomas. J Neurosurg. 133:1662–1670. 2020.
Article24. Roelz R, Reinacher P, Jabbarli R, Kraeutle R, Hippchen B, Egger K, et al. Surgical ventricular entry is a key risk factor for leptomeningeal metastasis of high grade gliomas. Sci Rep. 5:17758. 2015.
Article25. Roldán G, Chan J, Eliasziw M, Cairncross JG, Forsyth PA. Leptomeningeal disease in oligodendroglial tumors: a population-based study. J Neurooncol. 104:811–815. 2011.
Article26. Sreenivasan SA, Madhugiri VS, Sasidharan GM, Kumar RV. Measuring glioma volumes: a comparison of linear measurement based formulae with the manual image segmentation technique. J Cancer Res Ther. 12:161–168. 2016.
Article27. Suki D, Abouassi H, Patel AJ, Sawaya R, Weinberg JS, Groves MD. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg. 108:248–257. 2008.
Article28. Suki D, Hatiboglu MA, Patel AJ, Weinberg JS, Groves MD, Mahajan A, et al. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery. 64:664–676. discussion 674-976. 2009.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Leptomeningeal Metastasis Associated with Cerebral Venous Thrombosis
- Breast Cancer with Leptomeningeal Metastasis
- Diagnostic Value of Cerebrospinal Fluid Level of Carcinoembryonic Antigen in Patients with Leptomeningeal Carcinomatous Metastasis
- The Clinical Features of Spinal Leptomeningeal Dissemination from Malignant Gliomas
- Malignant Ascites after Subduroperitoneal Shunt in a Patient with Leptomeningeal Metastasis