J Neurocrit Care.
2023 Jun;16(1):18-27. 10.18700/jnc.220052.
Neuroprotective effects of chloroquine on neurological scores, blood-brain barrier permeability, and brain edema after traumatic brain injury in male rats
- Affiliations
-
- 1Department of Physiology and Pharmacology, Mazandaran University of Medical Sciences, Ramsar, Iran
- 2Department of Neuroscience, Faculty of Advanced Technologies in Medicine, Mazandaran University of Medical Sciences, Sari, Iran
- 3Immunogenetics Research Center, Department of Physiology, Mazandaran University of Medical Sciences, Sari, Iran
- 4Department of Biology, Islamic Azad University of Tonekabon, Tonekabon, Iran
- KMID: 2543388
- DOI: http://doi.org/10.18700/jnc.220052
Abstract
- Background
Traumatic brain injury (TBI) is one of leading causes of death among young people worldwide. Chloroquine, an antimalarial drug, has been shown to easily cross the blood-brain barrier (BBB) and inhibit autophagy in a variety of disorders, including Alzheimer disease and brain ischemia. We investigated the effects of chloroquine on neuronal protection after induction of brain trauma in male rats.
Methods
A total of 120 male Wistar rats were treated with chloroquine at doses of 1.5, 3, and 6 mg/kg intraperitoneally after induction of diffuse TBIs. The veterinary coma scale was used to assess short-term neurological deficits. BBB disruption was evaluated using the Evans Blue dye method 6-hour post-injury. Vestibulomotor function was evaluated using the beam walk and beam balance methods. Histopathological changes in the brain tissue in different groups were evaluated using light microscopy and hematoxylin-eosin staining. Brain water and cerebrospinal fluid (CSF) contents of matrix metalloproteinase 9 (MMP-9) were assessed using the wet/dry method and enzyme-linked immunosorbent assay, respectively.
Results
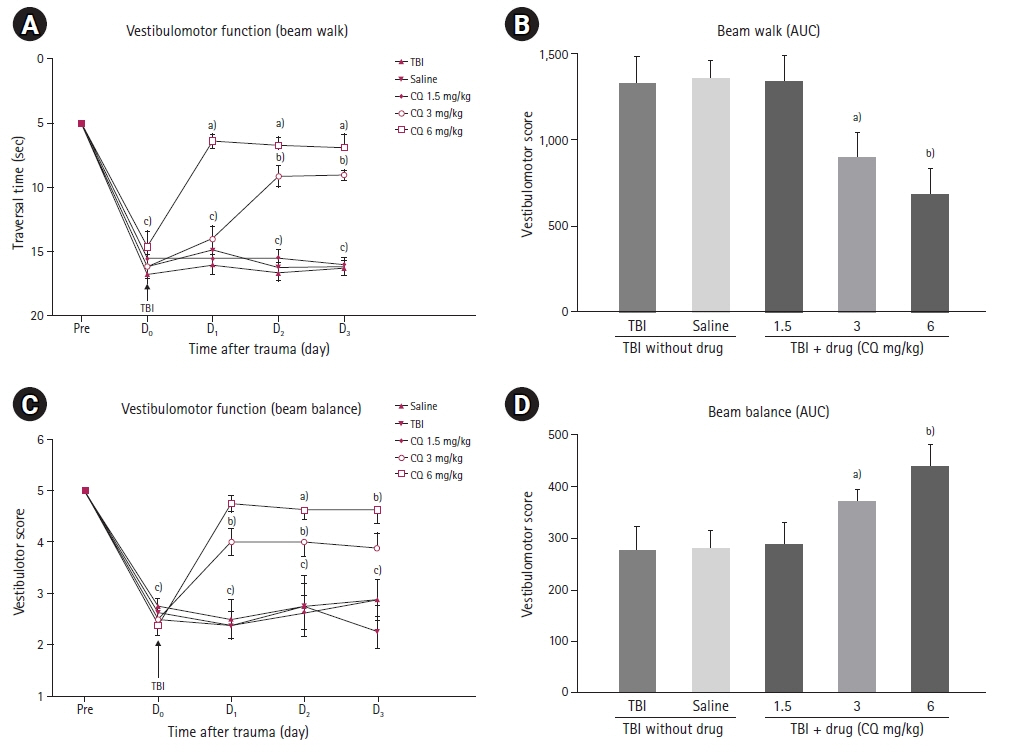
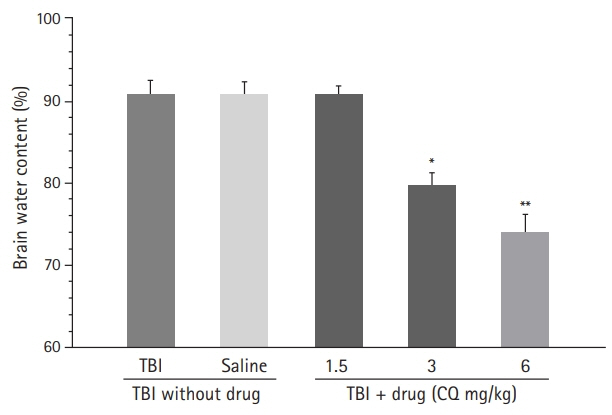
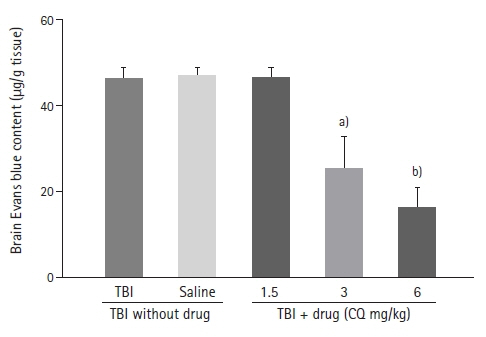
The results showed that injecting chloroquine (3 and 6 mg/kg) 30 minutes after TBI significantly reduced brain edema and BBB disruption, and recovered neurological deficits post-TBI (P<0.01). Furthermore, CSF MMP-9 was significantly reduced after administration of 1.5 mg/kg chloroquine (P<0.01).
Conclusion
Chloroquine has neuroprotective effects in the brain, and thus, has the potential to mitigate the effects of brain trauma. It is possible that the anti-inflammatory and neurogenic effects of chloroquine are due to a decrease in MMP secretion in the CSF.
Keyword
Figure
Reference
-
1. Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil. 2008; 23:123–31.2. Ouellet MC, Beaulieu-Bonneau S, Morin CM. Sleep-wake disturbances after traumatic brain injury. Lancet Neurol. 2015; 14:746–57.3. Landre N, Poppe CJ, Davis N, Schmaus B, Hobbs SE. Cognitive functioning and postconcussive symptoms in trauma patients with and without mild TBI. Arch Clin Neuropsychol. 2006; 21:255–73.4. Prins M, Greco T, Alexander D, Giza CC. The pathophysiology of traumatic brain injury at a glance. Dis Model Mech. 2013; 6:1307–15.5. Najem D, Rennie K, Ribecco-Lutkiewicz M, Ly D, Haukenfrers J, Liu Q, et al. Traumatic brain injury: classification, models, and markers. Biochem Cell Biol. 2018; 96:391–406.6. Setnik L, Bazarian JJ. The characteristics of patients who do not seek medical treatment for traumatic brain injury. Brain Inj. 2007; 21:1–9.7. Liu CL, Chen S, Dietrich D, Hu BR. Changes in autophagy after traumatic brain injury. J Cereb Blood Flow Metab. 2008; 28:674–83.8. Cui C, Wang C, Jin F, Yang M, Kong L, Han W, et al. Calcitriol confers neuroprotective effects in traumatic brain injury by activating Nrf2 signaling through an autophagy-mediated mechanism. Mol Med. 2021; 27:118.9. Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol. 2009; 66:378–89.10. Cui CM, Gao JL, Cui Y, Sun LQ, Wang YC, Wang KJ, et al. Chloroquine exerts neuroprotection following traumatic brain injury via suppression of inflammation and neuronal autophagic death. Mol Med Rep. 2015; 12:2323–8.11. Qin A, Zhang Q, Wang J, Sayeed I, Stein DG. Is a combination of progesterone and chloroquine more effective than either alone in the treatment of cerebral ischemic injury? Restor Neurol Neurosci. 2019; 37:1–10.12. Giovanella F, Ferreira GK, de Prá SD, Carvalho-Silva M, Gomes LM, Scaini G, et al. Effects of primaquine and chloroquine on oxidative stress parameters in rats. An Acad Bras Cienc. 2015; 87(2 Suppl):1487–96.13. Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006; 11:1696–701.14. Truettner JS, Alonso OF, Dietrich WD. Influence of therapeutic hypothermia on matrix metalloproteinase activity after traumatic brain injury in rats. J Cereb Blood Flow Metab. 2005; 25:1505–16.15. Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg. 1994; 80:291–300.16. Marmarou CR, Prieto R, Taya K, Young HF, Marmarou A. Marmarou weight drop injury model. In : Chen J, Xu ZC, Xu XM, Zhang JH, editors. Animal models of acute neurological injuries. Totowa, NJ: Humana Press;2009. p. 393–407.17. Foda MA, Marmarou A. A new model of diffuse brain injury in rats. Part II: morphological characterization. J Neurosurg. 1994; 80:301–13.18. Khaksari M, Soltani Z, Shahrokhi N, Moshtaghi G, Asadikaram G. The role of estrogen and progesterone, administered alone and in combination, in modulating cytokine concentration following traumatic brain injury. Can J Physiol Pharmacol. 2011; 89:31–40.19. Asl SZ, Khaksari M, Khachki AS, Shahrokhi N, Nourizade S. Contribution of estrogen receptors alpha and beta in the brain response to traumatic brain injury. J Neurosurg. 2013; 119:353–61.20. Khachaki AS, Haddad MK, Shahrokhi NA, Sepehri G. Effects of different phases of estrous cycle on brain edema and neurological outcomes after severe traumatic brain injury in female rats. Koomesh. 2011; 13:62–72.21. Rahimi S, Dadfar B, Tavakolian G, Asadi Rad A, Rashid Shabkahi A, Siahposht-Khachaki A. Morphine attenuates neuroinflammation and blood-brain barrier disruption following traumatic brain injury through the opioidergic system. Brain Res Bull. 2021; 176:103–11.22. Khaksari M, Maghool F, Asadikaram G, Hajializadeh Z. Effects of sex steroid hormones on neuromedin S and neuromedin U2 receptor expression following experimental traumatic brain injury. Iran J Basic Med Sci. 2016; 19:1080–9.23. Soltani Z, Khaksari M, Jafari E, Iranpour M, Shahrokhi N. Is genistein neuroprotective in traumatic brain injury? Physiol Behav. 2015; 152(Pt A):26–31.24. Rahimi S, Ferdowsi A, Siahposht-Khachaki A. Neuroprotective effects of metformin on traumatic brain injury in rats is associated with the AMP-activated protein kinase signaling pathway. Metab Brain Dis. 2020; 35:1135–44.25. Monaco CM, Mattiola VV, Folweiler KA, Tay JK, Yelleswarapu NK, Curatolo LM, et al. Environmental enrichment promotes robust functional and histological benefits in female rats after controlled cortical impact injury. Exp Neurol. 2013; 247:410–8.26. Nirogi R, Kandikere V, Mudigonda K, Bhyrapuneni G, Muddana N, Saralaya R, et al. A simple and rapid method to collect the cerebrospinal fluid of rats and its application for the assessment of drug penetration into the central nervous system. J Neurosci Methods. 2009; 178:116–9.27. Pegg CC, He C, Stroink AR, Kattner KA, Wang CX. Technique for collection of cerebrospinal fluid from the cisterna magna in rat. J Neurosci Methods. 2010; 187:8–12.28. Shamsi Meymandi M, Soltani Z, Sepehri G, Amiresmaili S, Farahani F, Moeini Aghtaei M. Effects of pregabalin on brain edema, neurologic and histologic outcomes in experimental traumatic brain injury. Brain Res Bull. 2018; 140:169–75.29. Lesiak A, Narbutt J, Sysa-Jedrzejowska A, Lukamowicz J, McCauliffe DP, Wózniacka A. Effect of chloroquine phosphate treatment on serum MMP-9 and TIMP-1 levels in patients with systemic lupus erythematosus. Lupus. 2010; 19:683–8.30. Hazra S, Chaudhuri AG, Tiwary BK, Chakrabarti N. Matrix metallopeptidase 9 as a host protein target of chloroquine and melatonin for immunoregulation in COVID-19: a network-based meta-analysis. Life Sci. 2020; 257:118096.31. Chen K, Sun Z. Chloroquine attenuates klotho gene deficiency‐induced arterial stiffening and hypertension by suppression of autophagy. FASEB J. 2016; 30:1206–12.32. Dai M, Reznik SE, Spray DC, Weiss LM, Tanowitz HB, Gulinello M, et al. Persistent cognitive and motor deficits after successful antimalarial treatment in murine cerebral malaria. Microbes Infect. 2010; 12:1198–207.33. Mielke JG, Murphy MP, Maritz J, Bengualid KM, Ivy GO. Chloroquine administration in mice increases beta-amyloid immunoreactivity and attenuates kainate-induced blood-brain barrier dysfunction. Neurosci Lett. 1997; 227:169–72.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Agmatine Attenuates Brain Edema and Apoptotic Cell Death after Traumatic Brain Injury
- The Effect of Hypertonic Saline and Mannitol against Edema Formation after Cryogenic Brain Injury in Rats
- Arterial Spin Labelling-Based Blood-Brain Barrier Assessment and Its Applications
- Superior Cervical Sympathetic Ganglion Block may not Influence Early Brain Damage Induced by Permanent Focal Cerebral Ischemia in Rats
- Effect of Selective Brain Cooling During the Cerebral Ischemia on the Post: Ischemic Brain Water Content in the Rabbit