Nutr Res Pract.
2023 Jun;17(3):475-486. 10.4162/nrp.2023.17.3.475.
Effect of preoperative immunonutrition on fecal microbiota in colon cancer patients: a secondary analysis of a randomized controlled trial
- Affiliations
-
- 1Department of Surgery, Chonnam National University Hwasun Hospital and Chonnam National University Medical School, Hwasun 58128, Korea
- KMID: 2542844
- DOI: http://doi.org/10.4162/nrp.2023.17.3.475
Abstract
- BACKGROUND/OBJECTIVES
This study aimed to evaluate the effect of preoperative immunonutrition on the composition of fecal microbiota following a colon cancer surgery.
MATERIALS/METHODS
This study was a secondary analysis of a randomized controlled trial assessing the impact of preoperative immunonutrition on the postoperative outcomes of colon cancer surgery. Patients with primary colon cancer were enrolled and randomly assigned to receive additional preoperative immunonutrition or a normal diet alone. Oral nutritional supplementation (400 mL/day) with arginine and ω-3 fatty acids were administered to patients in the immunonutrition group for 7 days prior to surgery. Thirtytwo fecal samples were collected from 16 patients in each group, and the composition of fecal microbiota was compared between the 2 groups.
RESULTS
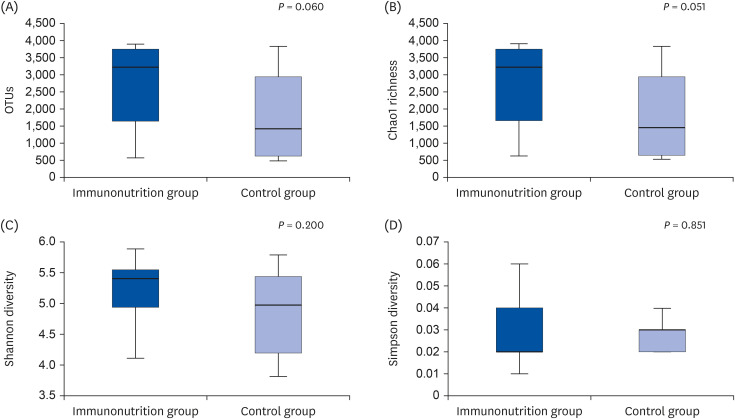
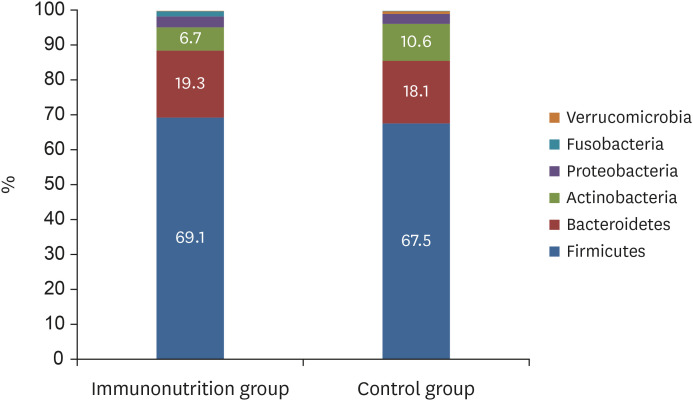
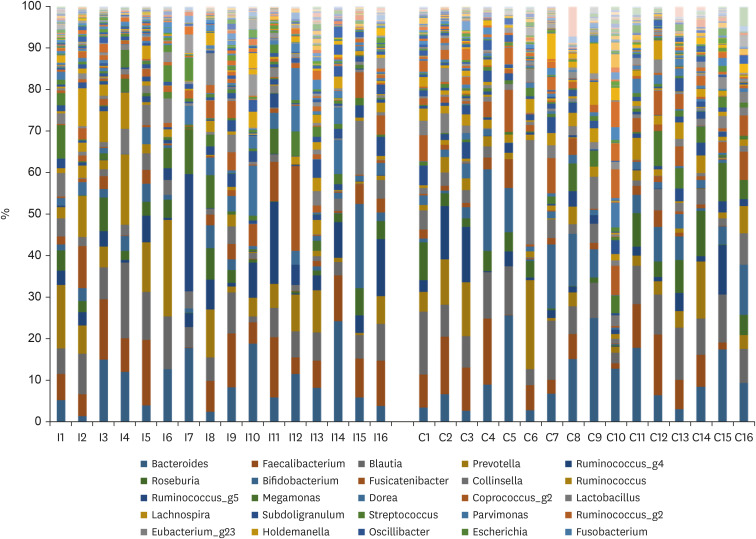
At the phylum level, no significant difference was observed in the composition of microbiota between the 2 groups (Firmicutes, 69.1% vs. 67.5%, P = 0.624; Bacteroidetes, 19.3% vs. 18.1%, P = 0.663; Actinobacteria, 6.7% vs. 10.6%, P = 0.080). The Firmicutes/Bacteroidetes ratio (4.43 ± 2.32 vs. 4.55 ± 2.51, P = 0.897) was also similar between the 2 groups. At the genus level, the proportions of beneficial bacteria such as Faecalibacterium spp. (8.1% vs. 6.4%, P = 0.328) and Prevotella spp. (6.9% vs. 4.8%, P = 0.331) were higher, while that of Clostridium spp. was lower (0.5% vs. 1.2%, P = 0.121) in the immunonutrition group, but the difference was not significant.
CONCLUSIONS
Immunonutrition showed no significant association with the composition of fecal microbiota. The relationship between immunonutrition and the fecal microbiota should be investigated further in large-scale studies.
Keyword
Figure
Reference
-
1. Cresci GA, Bawden E. Gut microbiome: what we do and don’t know. Nutr Clin Pract. 2015; 30:734–746. PMID: 26449893.2. Guyton K, Alverdy JC. The gut microbiota and gastrointestinal surgery. Nat Rev Gastroenterol Hepatol. 2017; 14:43–54. PMID: 27729657.3. Agnes A, Puccioni C, D’Ugo D, Gasbarrini A, Biondi A, Persiani R. The gut microbiota and colorectal surgery outcomes: facts or hype? A narrative review. BMC Surg. 2021; 21:83. PMID: 33579260.4. Koliarakis I, Athanasakis E, Sgantzos M, Mariolis-Sapsakos T, Xynos E, Chrysos E, Souglakos J, Tsiaoussis J. Intestinal microbiota in colorectal cancer surgery. Cancers (Basel). 2020; 12:3011. PMID: 33081401.5. Tourelle KM, Boutin S, Weigand MA, Schmitt FC. The association of gut microbiota and complications in gastrointestinal-cancer therapies. Biomedicines. 2021; 9:1305. PMID: 34680424.6. Keskey R, Papazian E, Lam A, Toni T, Hyoju S, Thewissen R, Zaborin A, Zaborina O, Alverdy JC. Defining microbiome readiness for surgery: dietary prehabilitation and stool biomarkers as predictive tools to improve outcome. Ann Surg. 2022; 276:e361–e369. PMID: 33156068.7. Darbandi A, Mirshekar M, Shariati A, Moghadam MT, Lohrasbi V, Asadolahi P, Talebi M. The effects of probiotics on reducing the colorectal cancer surgery complications: a periodic review during 2007-2017. Clin Nutr. 2020; 39:2358–2367. PMID: 31831184.8. Liu PC, Yan YK, Ma YJ, Wang XW, Geng J, Wang MC, Wei FX, Zhang YW, Xu XD, Zhang YC. Probiotics reduce postoperative infections in patients undergoing colorectal surgery: a systematic review and meta-analysis. Gastroenterol Res Pract. 2017; 2017:6029075. PMID: 28484489.9. Weimann A. Influence of nutritional status on postoperative outcome in patients with colorectal cancer - the emerging role of the microbiome. Innov Surg Sci. 2017; 3:55–64. PMID: 31579766.10. Lee SY, Lee J, Park HM, Kim CH, Kim HR. Impact of preoperative immunonutrition on the outcomes of colon cancer surgery: results from a randomized controlled trial. Ann Surg. Forthcoming. 2021.11. Probst P, Ohmann S, Klaiber U, Hüttner FJ, Billeter AT, Ulrich A, Büchler MW, Diener MK. Meta-analysis of immunonutrition in major abdominal surgery. Br J Surg. 2017; 104:1594–1608. PMID: 28940219.12. Fukatsu K. Role of nutrition in gastroenterological surgery. Ann Gastroenterol Surg. 2019; 3:160–168. PMID: 30923785.13. Lee SY, Yeom SS, Kim CH, Kim HR. Effect of preoperative immunonutrition on outcomes of colon cancer surgery: study protocol for a randomized controlled trial. Trials. 2020; 21:628. PMID: 32641081.14. Lee SY, Jung MR, Kim CH, Kim YJ, Kim HR. Nutritional risk screening score is an independent predictive factor of anastomotic leakage after rectal cancer surgery. Eur J Clin Nutr. 2018; 72:489–495. PMID: 29459787.15. Leylabadlo HE, Ghotaslou R, Feizabadi MM, Farajnia S, Moaddab SY, Ganbarov K, Khodadadi E, Tanomand A, Sheykhsaran E, Yousefi B, et al. The critical role of Faecalibacterium prausnitzii in human health: an overview. Microb Pathog. 2020; 149:104344. PMID: 32534182.16. Ferreira-Halder CV, Faria AV, Andrade SS. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract Res Clin Gastroenterol. 2017; 31:643–648. PMID: 29566907.17. Lapiere A, Geiger M, Robert V, Demarquay C, Auger S, Chadi S, Benadjaoud M, Fernandes G, Milliat F, Langella P, et al. Prophylactic Faecalibacterium prausnitzii treatment prevents the acute breakdown of colonic epithelial barrier in a preclinical model of pelvic radiation disease. Gut Microbes. 2020; 12:1–15.18. Damle RN, Cherng NB, Flahive JM, Davids JS, Maykel JA, Sturrock PR, Sweeney WB, Alavi K. Clostridium difficile infection after colorectal surgery: a rare but costly complication. J Gastrointest Surg. 2014; 18:1804–1811. PMID: 25091840.19. Lee J, Yeom SS, Lee SY, Kim CH, Kim HR, Kim YJ. The diagnostic delay and treatment outcome of Clostridium difficile infection in the patients who underwent rectal surgery. Korean J Clin Oncol. 2019; 15:34–39.20. Merra G, Noce A, Marrone G, Cintoni M, Tarsitano MG, Capacci A, De Lorenzo A. Influence of Mediterranean diet on human gut microbiota. Nutrients. 2020; 13:7. PMID: 33375042.21. Yang Q, Liang Q, Balakrishnan B, Belobrajdic DP, Feng QJ, Zhang W. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients. 2020; 12:381. PMID: 32023943.22. Moreno-Pérez D, Bressa C, Bailén M, Hamed-Bousdar S, Naclerio F, Carmona M, Pérez M, González-Soltero R, Montalvo-Lominchar MG, Carabaña C, et al. Effect of a protein supplement on the gut microbiota of endurance athletes: a randomized, controlled, double-blind pilot study. Nutrients. 2018; 10:337. PMID: 29534465.23. Schmitt FC, Schneider M, Mathejczyk W, Weigand MA, Figueiredo JC, Li CI, Shibata D, Siegel EM, Toriola AT, Ulrich CM, et al. Postoperative complications are associated with long-term changes in the gut microbiota following colorectal cancer surgery. Life (Basel). 2021; 11:246. PMID: 33809741.24. van Praagh JB, de Goffau MC, Bakker IS, van Goor H, Harmsen HJ, Olinga P, Havenga K. Mucus microbiome of anastomotic tissue during surgery has predictive value for colorectal anastomotic leakage. Ann Surg. 2019; 269:911–916. PMID: 29303807.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of preoperative immunonutrition in patients with colorectal cancer: a narrative review
- Fecal Microbiota Transplantation and the Brain Microbiota in Neurological Diseases
- Refractory Clostridium difficile Infection Cured With Fecal Microbiota Transplantation in Vancomycin-Resistant Enterococcus Colonized Patient
- Fecal Microbiota Transplantation to Patients with Refractory Very Early Onset Ulcerative Colitis
- Commensal Microbiota and Cancer Immunotherapy: Harnessing Commensal Bacteria for Cancer Therapy