Clin Endosc.
2023 May;56(3):353-366. 10.5946/ce.2022.021.
Pancreatic duct lavage cytology combined with a cell-block method for patients with possible pancreatic ductal adenocarcinomas, including pancreatic carcinoma in situ
- Affiliations
-
- 1Department of Gastroenterology, Sendai City Medical Center, Sendai, Japan
- 2Department of Surgery, Sendai City Medical Center, Sendai, Japan
- 3Department of Pathology, Sendai City Medical Center, Sendai, Japan
- 4Department of Gastroenterology, Yokohama City University Medical Center, Yokohama, Japan
- KMID: 2542463
- DOI: http://doi.org/10.5946/ce.2022.021
Abstract
- Background/Aims
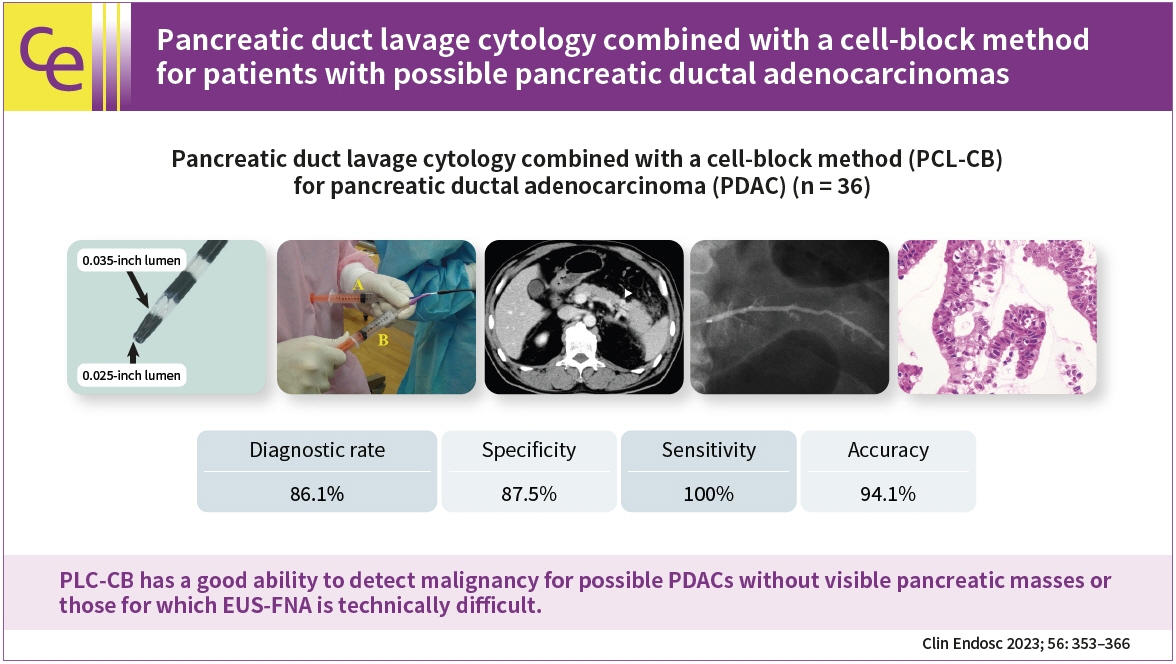
This study aimed to clarify the efficacy and safety of pancreatic duct lavage cytology combined with a cell-block method (PLC-CB) for possible pancreatic ductal adenocarcinomas (PDACs).
Methods
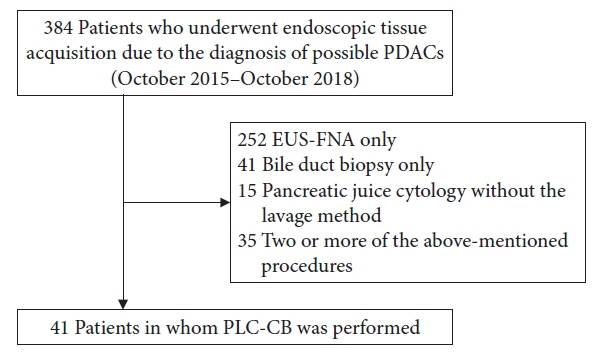
This study included 41 patients with suspected PDACs who underwent PLC-CB mainly because they were unfit for undergoing endoscopic ultrasonography-guided fine needle aspiration. A 6-Fr double lumen catheter was mainly used to perform PLC-CB. Final diagnoses were obtained from the findings of resected specimens or clinical outcomes during surveillance after PLC-CB.
Results
Histocytological evaluations using PLC-CB were performed in 87.8% (36/41) of the patients. For 31 of the 36 patients, final diagnoses (invasive PDAC, 12; pancreatic carcinoma in situ, 5; benignancy, 14) were made, and the remaining five patients were excluded due to lack of surveillance periods after PLC-CB. For 31 patients, the sensitivity, specificity, and accuracy of PLC-CB for detecting malignancy were 94.1%, 100%, and 96.8%, respectively. In addition, they were 87.5%, 100%, and 94.1%, respectively, in 17 patients without pancreatic masses detectable using endoscopic ultrasonography. Four patients developed postprocedural pancreatitis, which improved with conservative therapy.
Conclusions
PLC-CB has an excellent ability to detect malignancies in patients with possible PDACs, including pancreatic carcinoma in situ.
Keyword
Figure
Reference
-
1. ASGE Standards of Practice Committee, Eloubeidi MA, Decker GA, et al. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest Endosc. 2016; 83:17–28.2. Renelus BD, Jamorabo DS, Boston I, et al. Endoscopic ultrasound-guided fine needle biopsy needles provide higher diagnostic yield compared to endoscopic ultrasound-guided fine needle aspiration needles when sampling solid pancreatic lesions: a meta-analysis. Clin Endosc. 2021; 54:261–268.3. Chung MJ, Park SW, Kim SH, et al. Clinical and technical guideline for endoscopic ultrasound (EUS)-guided tissue acquisition of pancreatic solid tumor: Korean Society of Gastrointestinal Endoscopy (KSGE). Clin Endosc. 2021; 54:161–181.4. Nakaizumi A, Tatsuta M, Uehara H, et al. Usefulness of simple endoscopic aspiration cytology of pancreatic juice for diagnosis of early pancreatic neoplasm: a prospective study. Dig Dis Sci. 1997; 42:1796–1803.5. Koshita S, Noda Y, Ito K, et al. Pancreatic juice cytology with immunohistochemistry to detect malignancy and histologic subtypes in patients with branch duct type intraductal papillary mucinous neoplasms of the pancreas. Gastrointest Endosc. 2017; 85:1036–1046.6. Noda Y, Fujita N, Kobayashi G, et al. Prospective randomized controlled study comparing cell block method and conventional smear method for pancreatic juice cytology. Dig Endosc. 2012; 24:168–174.7. Koshita S, Noda Y, Kanno Y, et al. Value of repeated cytology for intraductal papillary mucinous neoplasms of the pancreas with high risk potential of malignancy: is it a promising method for monitoring a malignant transformation? Pancreatology. 2020; 20:1164–1174.8. Sai JK, Nobukawa B, Matsumura Y, et al. Pancreatic duct lavage cytology with the cell block method for discriminating benign and malignant branch-duct type intraductal papillary mucinous neoplasms. Gastrointest Endosc. 2013; 77:726–735.9. Kanno Y, Koshita S, Ogawa T, et al. Predictive value of localized stenosis of the main pancreatic duct for early detection of pancreatic cancer. Clin Endosc. 2019; 52:588–597.10. Noda Y, Fujita N, Kobayashi G, et al. Diagnostic efficacy of the cell block method in comparison with smear cytology of tissue samples obtained by endoscopic ultrasound-guided fine-needle aspiration. J Gastroenterol. 2010; 45:868–875.11. Guidelines of the Papanicolaou Society of Cytopathology for fine-needle aspiration procedure and reporting. Diagn Cytopathol. 1997; 17:239–247.12. Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991; 37:383–393.13. Iiboshi T, Hanada K, Fukuda T, et al. Value of cytodiagnosis using endoscopic nasopancreatic drainage for early diagnosis of pancreatic cancer: establishing a new method for the early detection of pancreatic carcinoma in situ. Pancreas. 2012; 41:523–529.14. Satoh T, Kikuyama M, Kawaguchi S, et al. Acute pancreatitis-onset carcinoma in situ of the pancreas with focal fat replacement diagnosed using serial pancreatic-juice aspiration cytologic examination (SPACE). Clin J Gastroenterol. 2017; 10:541–545.15. Peters ML, Eckel A, Mueller PP, et al. Progression to pancreatic ductal adenocarcinoma from pancreatic intraepithelial neoplasia: results of a simulation model. Pancreatology. 2018; 18:928–934.16. Mikata R, Ishihara T, Tada M, et al. Clinical usefulness of repeated pancreatic juice cytology via endoscopic naso-pancreatic drainage tube in patients with pancreatic cancer. J Gastroenterol. 2013; 48:866–873.17. Mouri T, Sasaki T, Serikawa M, et al. A comparison of 4-Fr with 5-Fr endoscopic nasopancreatic drainage catheters: a randomized, controlled trial. J Gastroenterol Hepatol. 2016; 31:1783–1789.18. Ito K, Fujita N, Noda Y, et al. Can pancreatic duct stenting prevent post-ERCP pancreatitis in patients who undergo pancreatic duct guidewire placement for achieving selective biliary cannulation? A prospective randomized controlled trial. J Gastroenterol. 2010; 45:1183–1191.19. Dumonceau JM, Andriulli A, Elmunzer BJ, et al. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) guideline - updated June 2014. Endoscopy. 2014; 46:799–815.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cell block created from pancreatic duct lavage is another jigsaw puzzle to diagnose early pancreatic ductal adenocarcinoma
- Pathological Classification of Panaeatic Cancer and Precancerous Casion
- Expression of S100A4 in Invasive Adenocarcinoma and Intraductal Papillary Mucinous Neoplasm of the Pancreas
- A Small Pancreatic Neuroendocrine Tumor with Marked Pancreatic Duct Dilatation and Parenchymal Atrophy
- A Case of Cutaneous Metastatic Adenosquamous Carcinoma of the Pancreas