Clin Endosc.
2023 May;56(3):315-324. 10.5946/ce.2022.072.
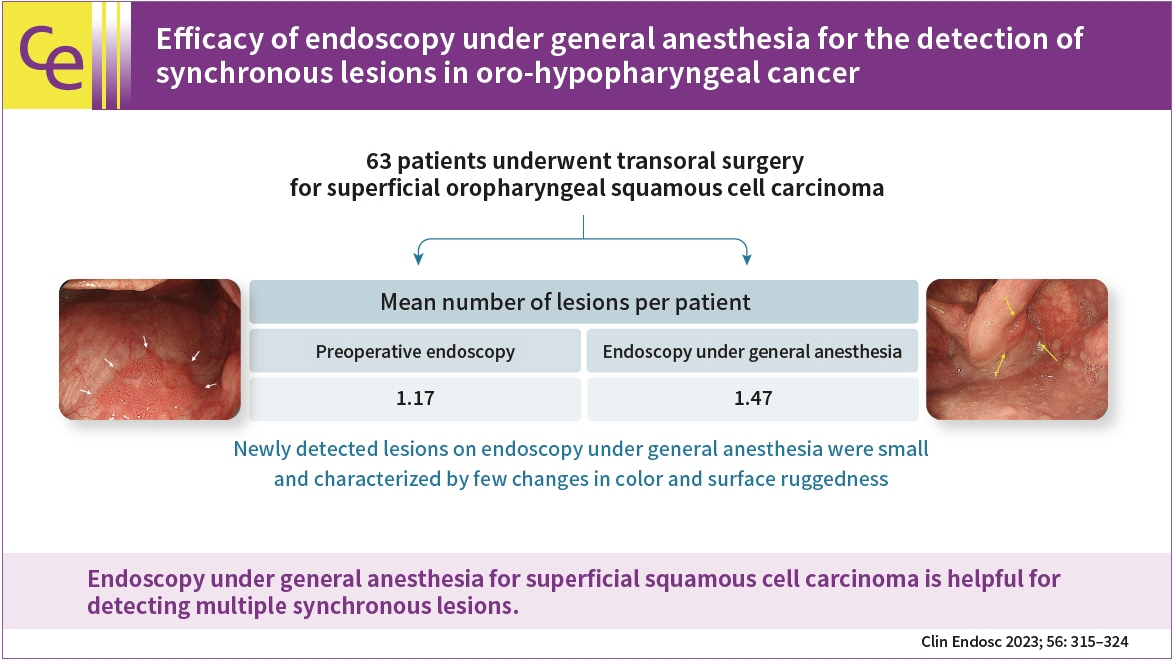
Efficacy of endoscopy under general anesthesia for the detection of synchronous lesions in oro-hypopharyngeal cancer
- Affiliations
-
- 1Department of Gastroenterology, Fukuoka University Chikushi Hospital, Fukuoka, Japan
- 2Department of Endoscopy, Fukuoka University Chikushi Hospital, Fukuoka, Japan
- 3Department of Gastroenterology, Ashiya Central Hospital, Fukuoka, Japan
- 4Department of Pathology, Fukuoka University Chikushi Hospital, Fukuoka, Japan
- 5Department of Otorhinolaryngology, Fukuoka University Chikushi Hospital, Fukuoka, Japan
- 6Department of Anesthesiology, Fukuoka University Chikushi Hospital, Fukuoka, Japan
- KMID: 2542459
- DOI: http://doi.org/10.5946/ce.2022.072
Abstract
- Background/Aims
Image-enhanced endoscopy can detect superficial oro-hypopharyngeal squamous cell carcinoma; however, reliable endoscopy of the pharyngeal region is challenging. Endoscopy under general anesthesia during transoral surgery occasionally reveals multiple synchronous lesions that remained undetected on preoperative endoscopy. Therefore, we aimed to determine the lesion detection capability of endoscopy under general anesthesia for superficial oro-hypopharyngeal squamous cell carcinoma.
Methods
This retrospective study included 63 patients who underwent transoral surgery for superficial oropharyngeal squamous cell carcinoma between April 2005 and December 2020. The primary endpoint was to compare the lesion detection capabilities of preoperative endoscopy and endoscopy under general anesthesia. Other endpoints included the comparison of clinicopathological findings between lesions detected using preoperative endoscopy and those newly detected using endoscopy under general anesthesia.
Results
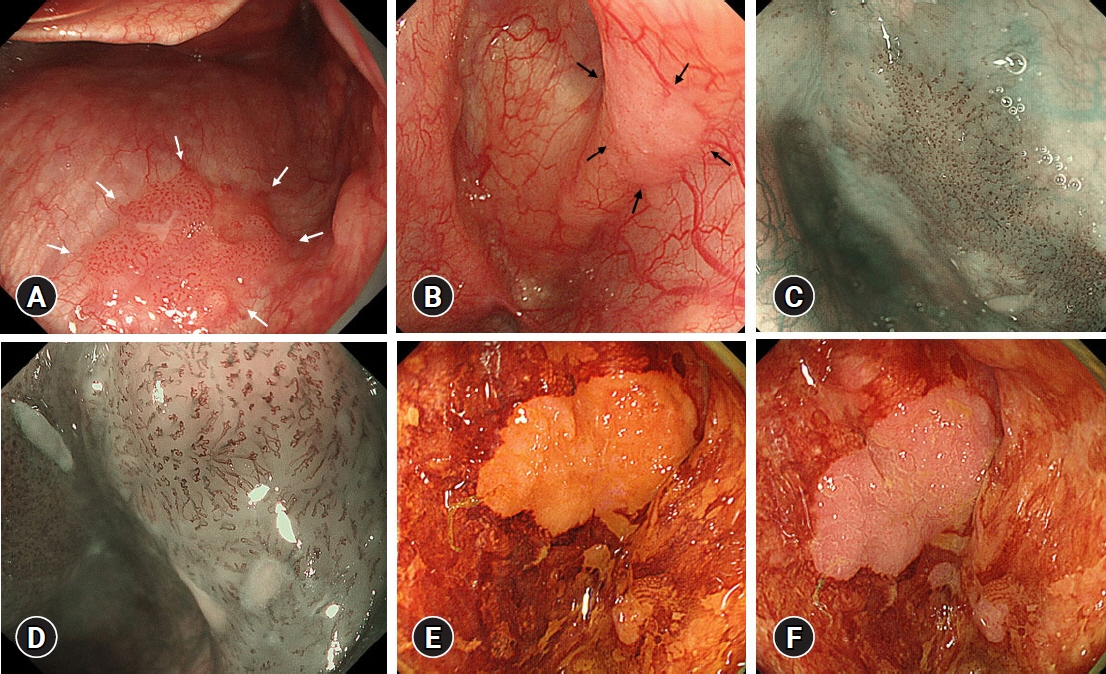
Fifty-eight patients (85 lesions) were analyzed. The mean number of lesions per patient detected was 1.17 for preoperative endoscopy and 1.47 for endoscopy under general anesthesia. Endoscopy under general anesthesia helped detect more lesions than preoperative endoscopy did (p<0.001). The lesions that were newly detected on endoscopy under general anesthesia were small and characterized by few changes in color and surface ruggedness.
Conclusions
Endoscopy under general anesthesia for superficial squamous cell carcinoma is helpful for detecting multiple synchronous lesions.
Keyword
Figure
Reference
-
1. Matsubara T, Yamada K, Nakagawa A. Risk of second primary malignancy after esophagectomy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003; 21:4336–4341.2. Sato Y, Motoyama S, Maruyama K, et al. A second malignancy is the major cause of death among thoracic squamous cell esophageal cancer patients negative for lymph node involvement. J Am Coll Surg. 2005; 201:188–193.3. Kennedy WR, Amdur RJ, Boyce BJ, et al. Neck management with total laryngectomy and adjuvant radiotherapy in locally advanced larynx cancer. Oncol Res Treat. 2017; 40:503–506.4. Muto M, Nakane M, Katada C, et al. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004; 101:1375–1381.5. Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953; 6:963–968.6. Bova R, Goh R, Poulson M, et al. Total pharyngolaryngectomy for squamous cell carcinoma of the hypopharynx: a review. Laryngoscope. 2005; 115:864–869.7. Chao KS, Deasy JO, Markman J, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: initial results. Int J Radiat Oncol Biol Phys. 2001; 49:907–916.8. Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010; 28:1566–1572.9. Muto M, Satake H, Yano T, et al. Long-term outcome of transoral organ-preserving pharyngeal endoscopic resection for superficial pharyngeal cancer. Gastrointest Endosc. 2011; 74:477–484.10. Hanaoka N, Ishihara R, Takeuchi Y, et al. Endoscopic submucosal dissection as minimally invasive treatment for superficial pharyngeal cancer: a phase II study (with video). Gastrointest Endosc. 2015; 82:1002–1008.11. Katada C, Muto M, Fujii S, et al. Transoral surgery for superficial head and neck cancer: National Multi-Center Survey in Japan. Cancer Med. 2021; 10:3848–3861.12. Shimizu Y, Omori T, Yokoyama A, et al. Endoscopic diagnosis of early squamous neoplasia of the esophagus with iodine staining: high-grade intra-epithelial neoplasia turns pink within a few minutes. J Gastroenterol Hepatol. 2008; 23:546–550.13. Ishihara R, Kanzaki H, Iishi H, et al. Pink-color sign in esophageal squamous neoplasia, and speculation regarding the underlying mechanism. World J Gastroenterol. 2013; 19:4300–4308.14. Inoue H, Endo M, Takeshita K, et al. A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC). Surg Endosc. 1992; 6:264–265.15. The Japan Society for Head and Neck Cancer. General rules for clinical studies on head and neck cancer. The 6th ed, revised version. Kanehara & Co., Ltd;2019. Japanese.16. Wahlberg PC, Andersson KE, Biörklund AT, et al. Carcinoma of the hypopharynx: analysis of incidence and survival in Sweden over a 30-year period. Head Neck. 1998; 20:714–719.17. Kinjo Y, Nonaka S, Oda I, et al. The short-term and long-term outcomes of the endoscopic resection for the superficial pharyngeal squamous cell carcinoma. Endosc Int Open. 2015; 3:E266–E273.18. Yamaguchi H, Sato H, Tsukahara K, et al. Co-treatment with endoscopic laryngopharyngeal surgery and endoscopic submucosal dissection. Auris Nasus Larynx. 2021; 48:457–463.19. Morimoto H, Yano T, Yoda Y, et al. Clinical impact of surveillance for head and neck cancer in patients with esophageal squamous cell carcinoma. World J Gastroenterol. 2017; 23:1051–1058.20. Yamasaki Y, Ishihara R, Hanaoka N, et al. Pethidine hydrochloride is a better sedation method for pharyngeal observation by transoral endoscopy compared with no sedation and midazolam. Dig Endosc. 2017; 29:39–48.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopy under general anesthesia for detecting synchronous lesions of head and neck squamous cell carcinoma
- Endoscopic Resection of Hypopharyngeal Squamous Cell Carcinoma
- A Case of Endoscopic Removal of Hypopharyngeal Papilloma under General Anesthesia with Nasotracheal Intubation
- Endoscopic Treatment of Benign Hypopharyngeal Tumors
- Incidentally Detected Hypopharyngeal Mass during Endotracheal Intubation