Ann Surg Treat Res.
2023 May;104(5):288-295. 10.4174/astr.2023.104.5.288.
A nationwide study of compliance of venoactive drugs in chronic venous disease patients
- Affiliations
-
- 1Department of Surgery, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea
- KMID: 2542236
- DOI: http://doi.org/10.4174/astr.2023.104.5.288
Abstract
- Purpose
Venoactive drugs are widely used to improve the symptoms and signs of chronic venous disease. This study aimed to analyze the rate of adverse events after venoactive drug prescription and subsequent compliance and switching rates.
Methods
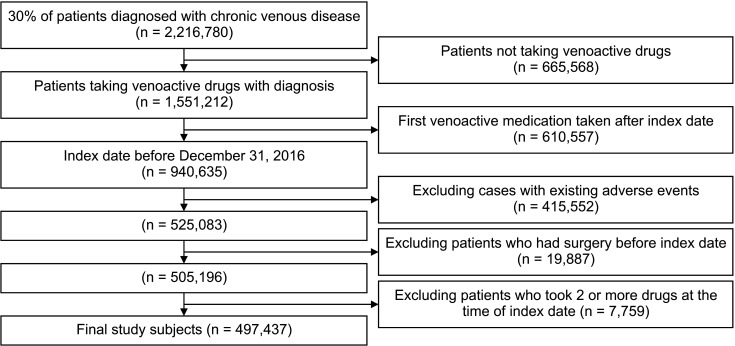
Using the National Health Insurance Service database, individuals with at least one chronic venous disease code between January 2009 and December 2019 were identified, and 30% (2,216,780 individuals) of these were sampled. Finally, 1,551,212 patients were included, and we analyzed adverse events, compliance, and switching rates with 8 venoactive drugs, including Vitis vinifera extract, naftazone, micronized purified flavonoid fraction, Vitis vinifera leaf extract, diosmin, diobsilate calcium, bilberry fruit dried extract, and sulodexide.
Results
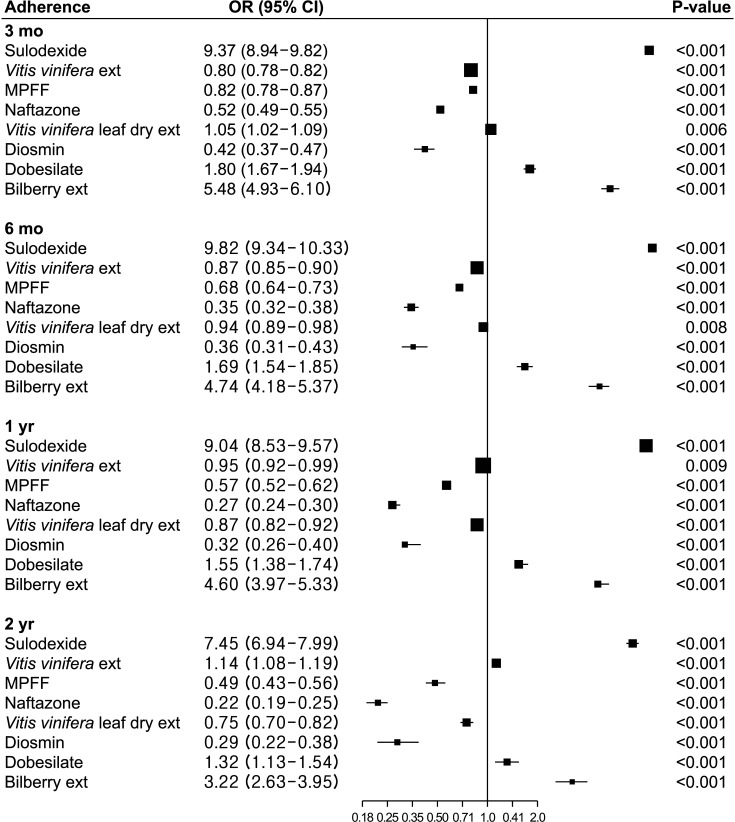
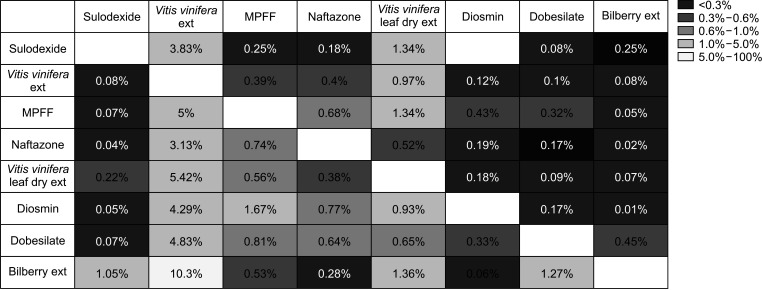
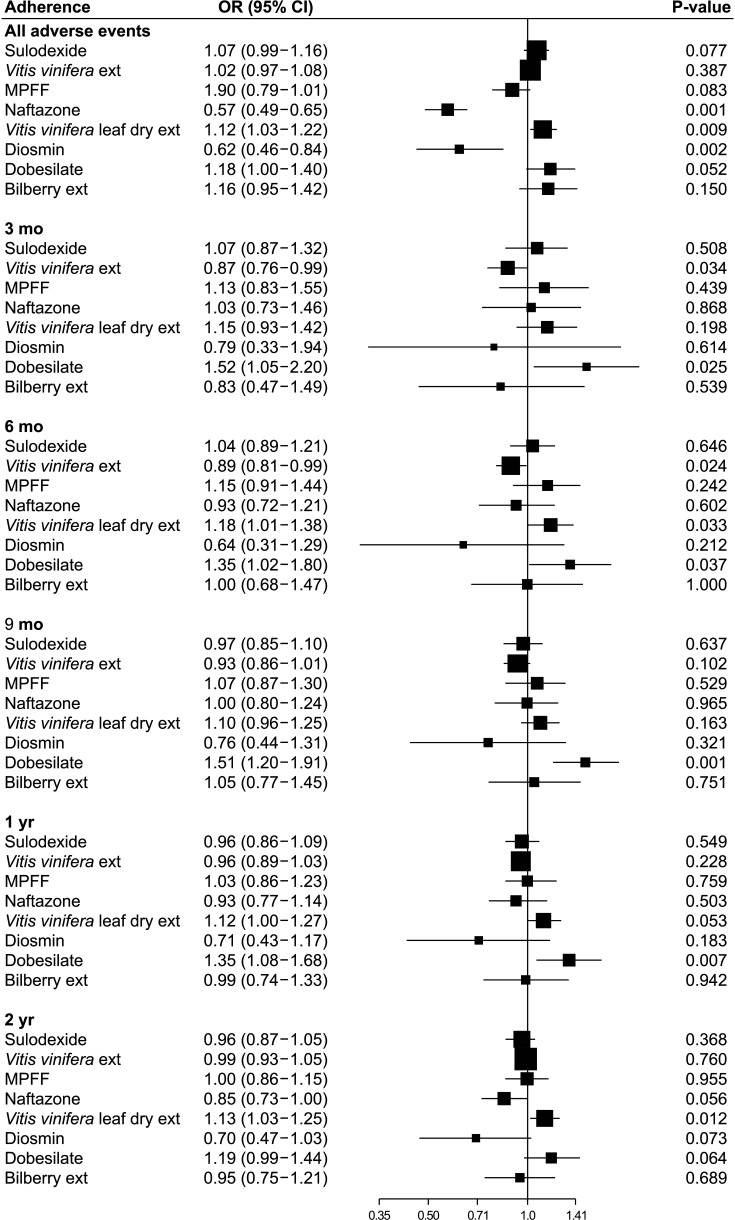
The most commonly prescribed venoactive drug was Vitis vinifera extract (72.2%), followed by sulodexide (9.3%), and Vitis vinifera leaf dry extract (8.2%). Adverse event rates were significantly lower in the naftazone and diosmin groups (P = 0.001 and P = 0.002, respectively) and significantly higher in the Vitis vinifera leaf dry extract group (P = 0.009). Drug adherence to sulodexide was the highest throughout the study period, followed by billberry extract and dobesilate (all P < 0.001). For most drugs, the drug switching rate was low (<5.0%).
Conclusion
Vitis vinifera extract was the most commonly prescribed venoactive drug in Korea, and drug adherence to sulodexide was the highest among all venoactive drugs. The adverse event rates were significantly lower in the naftazone and diosmin groups.
Keyword
Figure
Reference
-
1. De Maeseneer MG, Kakkos SK, Aherne T, Baekgaard N, Black S, Blomgren L, et al. Editor’s choice - European Society for Vascular Surgery (ESVS) 2022 clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur J Vasc Endovasc Surg. 2022; 63:184–267. PMID: 35027279.2. Dialsingh I, Mohammed SR, Medford RS, Budhoo E, White-Gittens I, Maharaj D. Conservative therapy significantly reduces patients’ chronic venous disease symptoms: a Caribbean insight into the VEIN Act Program. Phlebology. 2022; 37:651–661. PMID: 35848710.3. Martinez-Zapata MJ, Vernooij RW, Simancas-Racines D, Uriona Tuma SM, Stein AT, Moreno Carriles RM, et al. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev. 2020; 11:CD003229. PMID: 33141449.4. Cazaubon M, Benigni JP, Steinbruch M, Jabbour V, Gouhier-Kodas C. Is there a difference in the clinical efficacy of diosmin and micronized purified flavonoid fraction for the treatment of chronic venous disorders? Review of available evidence. Vasc Health Risk Manag. 2021; 17:591–600. PMID: 34556990.5. Ortega MA, Fraile-Martínez O, García-Montero C, Álvarez-Mon MA, Chaowen C, Ruiz-Grande F, et al. Understanding chronic venous disease: a critical overview of its pathophysiology and medical management. J Clin Med. 2021; 10:3239. PMID: 34362022.6. Mansilha A, Sousa J. Pathophysiological mechanisms of chronic venous disease and implications for venoactive drug therapy. Int J Mol Sci. 2018; 19:1669. PMID: 29874834.7. Sheikh P, Lohsiriwat V, Shelygin Y. Micronized purified flavonoid fraction in hemorrhoid disease: a systematic review and meta-analysis. Adv Ther. 2020; 37:2792–2812. PMID: 32399811.8. Perrin M, Ramelet AA. Pharmacological treatment of primary chronic venous disease: rationale, results and unanswered questions. Eur J Vasc Endovasc Surg. 2011; 41:117–125. PMID: 21126890.9. Allain H, Ramelet AA, Polard E, Bentué-Ferrer D. Safety of calcium dobesilate in chronic venous disease, diabetic retinopathy and haemorrhoids. Drug Saf. 2004; 27:649–660. PMID: 15230646.10. Rabe E, Stücker M, Esperester A, Schäfer E, Ottillinger B. Efficacy and tolerability of a red-vine-leaf extract in patients suffering from chronic venous insufficiency: results of a double-blind placebo-controlled study. Eur J Vasc Endovasc Surg. 2011; 41:540–547. PMID: 21239190.11. Belcaro G. A clinical comparison of pycnogenol, antistax, and stocking in chronic venous insufficiency. Int J Angiol. 2015; 24:268–274. PMID: 26648668.12. Nicolaides A, Kakkos S, Baekgaard N, Comerota A, de Maeseneer M, Eklof B, et al. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Part I. Int Angiol. 2018; 37:181–254. PMID: 29871479.13. Kalus U, Koscielny J, Grigorov A, Schaefer E, Peil H, Kiesewetter H. Improvement of cutaneous microcirculation and oxygen supply in patients with chronic venous insufficiency by orally administered extract of red vine leaves AS 195: a randomised, double-blind, placebo-controlled, crossover study. Drugs R D. 2004; 5:63–71. PMID: 15293865.14. Ciapponi A, Laffaire E, Roqué M. Calcium dobesilate for chronic venous insufficiency: a systematic review. Angiology. 2004; 55:147–154. PMID: 15026869.15. Senra Barros B, Kakkos SK, De Maeseneer M, Nicolaides AN. Chronic venous disease: from symptoms to microcirculation. Int Angiol. 2019; 38:211–218. PMID: 31112025.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medication Compliance in Psychiatric Outpatients of a University Hospital
- Chronic Venous Disease is a Progressive Disease that Requires Early Intervention
- The “C0s” Patient, What Do We Have to Know?
- Cases of Venous Stent Failure in Lower Extremities
- Structural Model of Hospital Nurses’ Compliance with Antineoplastic Drugs Safety Management Guidelines Based on Theory of Planned Behavior