Obstet Gynecol Sci.
2023 May;66(3):198-207. 10.5468/ogs.22262.
A personalized nomogram for predicting 3-year overall survival of patients with uterine carcinosarcoma in a tertiary care hospital in Southern Thailand
- Affiliations
-
- 1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand
- KMID: 2542226
- DOI: http://doi.org/10.5468/ogs.22262
Abstract
Objective
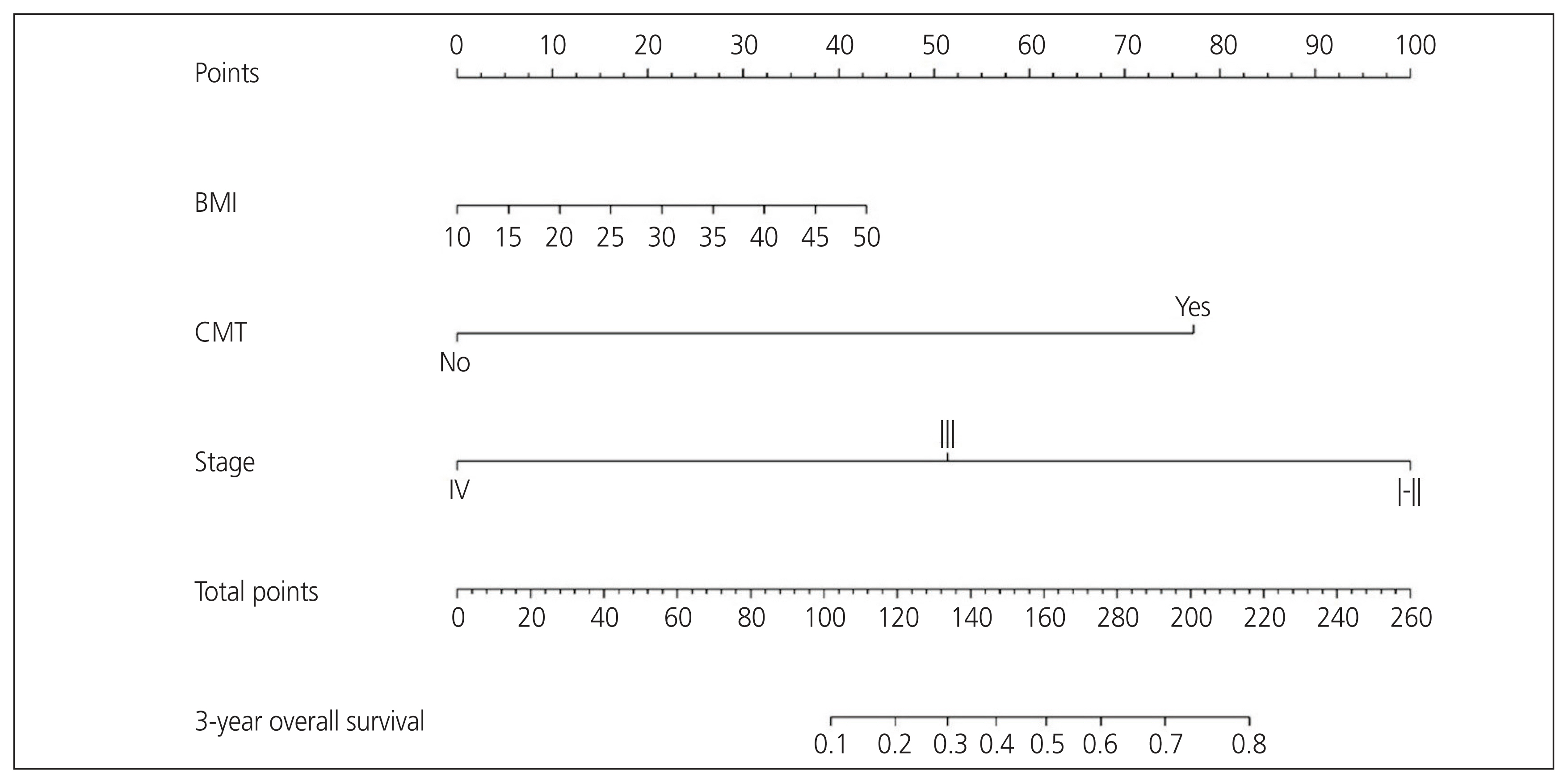
To develop a nomogram for predicting 3-year overall survival (OS) and outcomes of surgically staged patients with uterine carcinosarcomas (UCS).
Methods
This retrospective study analyzed the clinicopathological characteristics, treatment data, and oncological outcomes of 69 patients diagnosed with UCS between January 2002 and September 2018. Significant prognostic factors for OS were identified and integrated to develop a nomogram. Concordance probability (CP) was used as a precision measure. The model was internally validated using bootstrapping samples to correct overfitting.
Results
The median follow-up time was 19.4 months (range, 0.77-106.13 months). The 3-year OS was 41.8% (95% confidence interval [CI], 29.9-58.3%). The International Federation of Gynecology and Obstetrics (FIGO) stage and adjuvant chemotherapy were independent factors for OS. The CP of the nomogram integrating with body mass index (BMI), FIGO stage, and adjuvant chemotherapy was 0.72 (95% CI, 0.70-0.75). In addition, the calibration curves for the probability of 3-year OS demonstrated good agreement between the nomogram-predicted and observed data.
Conclusion
The established nomogram using BMI, FIGO stage, and adjuvant chemotherapy accurately predicted the 3-year OS of patients with UCS. The nomogram was useful for patient counselling and deciding on follow-up strategies.
Keyword
Figure
Reference
-
References
1. Cimbaluk D, Rotmensch J, Scudiere J, Gown A, Bitterman P. Uterine carcinosarcoma: immunohistochemical studies on tissue microarrays with focus on potential therapeutic targets. Gynecol Oncol. 2007; 105:138–44.
Article2. Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs [Internet]. Lyon: IARC Publications;c2014. [cited 2022 Mar 9]. Available from: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Female-Reproductive-Organs-2014 .3. Mutch DG. The new FIGO staging system for cancers of the vulva, cervix, endometrium and sarcomas. Gynecol Oncol. 2009; 3:325–8.
Article4. Galaal K, van der Heijden E, Godfrey K, Naik R, Kucukmetin A, Bryant A, et al. Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma. Cochrane Database Syst Rev. 2013; 2013:CD006812.
Article5. Saotome K, Yamagami W, Machida H, Ebina Y, Kobayashi Y, Tabata T, et al. Impact of lymphadenectomy on the treatment of endometrial cancer using data from the JSOG cancer registry. Obstet Gynecol Sci. 2021; 64:80–9.
Article6. Rovirosa A, Ascaso C, Arenas M, Ríos I, Del Pino M, Ordi J, et al. Pathologic prognostic factors in stage I-III uterine carcinosarcoma treated with postoperative radiotherapy. Arch Gynecol Obstet. 2014; 290:329–34.
Article7. Abdulfatah E, Lordello L, Khurram M, Van de Vijver K, Alosh B, Bandyopadhyay S, et al. Predictive histologic factors in carcinosarcomas of the uterus: a multi-institutional study. Int J Gynecol Pathol. 2019; 38:205–15.8. Cantrell LA, Blank SV, Duska LR. Uterine carcinosarcoma: a review of the literature. Gynecol Oncol. 2015; 137:581–8.
Article9. Ferguson SE, Tornos C, Hummer A, Barakat RR, Soslow RA. Prognostic features of surgical stage I uterine carcinosarcoma. Am J Surg Pathol. 2007; 31:1653–61.
Article10. AlHilli MM, Mariani A, Bakkum-Gamez JN, Dowdy SC, Weaver AL, Peethambaram PP, et al. Risk-scoring models for individualized prediction of overall survival in low-grade and high-grade endometrial cancer. Gynecol Oncol. 2014; 133:485–93.
Article11. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015; 16:e173–80.
Article12. Ouldamer L, Bendifallah S, Body G, Touboul C, Graesslin O, Raimond E, et al. Predicting poor prognosis recurrence in women with endometrial cancer: a nomogram developed by the FRANCOGYN study group. Br J Cancer. 2016; 115:1296–303.
Article13. Abu-Rustum NR, Zhou Q, Gomez JD, Alektiar KM, Hensley ML, Soslow RA, et al. A nomogram for predicting overall survival of women with endometrial cancer following primary therapy: toward improving individualized cancer care. Gynecol Oncol. 2010; 116:399–403.
Article14. Zivanovic O, Jacks LM, Iasonos A, Leitao MM Jr, Soslow RA, Veras E, et al. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer. 2012; 118:660–9.
Article15. Abu-Rustum NR, Iasonos A, Zhou Q, Oke E, Soslow RA, Alektiar KM, et al. Is there a therapeutic impact to regional lymphadenectomy in the surgical treatment of endometrial carcinoma? Am J Obstet Gynecol. 2008; 198:457e1–5.
Article16. Capanu M, Gönen M. Building a nomogram for survey-weighted cox models using R. J Stat Softw. 2015; 64:1–17.17. Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982; 247:2543–6.
Article18. Gonzalez Bosquet J, Terstriep SA, Cliby WA, Brown-Jones M, Kaur JS, Podratz KC, et al. The impact of multi-modal therapy on survival for uterine carcinosarcomas. Gynecol Oncol. 2010; 116:419–23.
Article19. Chen X, Arend R, Hamele-Bena D, Tergas AI, Hawver M, Tong GX, et al. Uterine carcinosarcomas: clinical, histopathologic and immunohistochemical characteristics. Int J Gynecol Pathol. 2017; 36:412–9.20. Harano K, Hirakawa A, Yunokawa M, Nakamura T, Satoh T, Nishikawa T, et al. Prognostic factors in patients with uterine carcinosarcoma: a multi-institutional retrospective study from the Japanese Gynecologic Oncology Group. Int J Clin Oncol. 2016; 21:168–76.
Article21. Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010; 19:3119–30.
Article22. Secord AA, Hasselblad V, Von Gruenigen VE, Gehrig PA, Modesitt SC, Bae-Jump V, et al. Body mass index and mortality in endometrial cancer: a systematic review and meta-analysis. Gynecol Oncol. 2016; 140:184–90.
Article23. Münstedt K, Wagner M, Kullmer U, Hackethal A, Franke FE. Influence of body mass index on prognosis in gynecological malignancies. Cancer Causes Control. 2008; 19:909–16.
Article24. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005; 9(Suppl 2):S51–63.
Article25. Leath CA 3rd, Numnum TM, Kendrick JE 4th, Frederick PJ, Rocconi RP, Conner MG, et al. Patterns of failure for conservatively managed surgical stage I uterine carcinosarcoma: implications for adjuvant therapy. Int J Gynecol Cancer. 2009; 19:888–91.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nomogram for Predicting Survival for Oral Squamous Cell Carcinoma
- Long-term recurrence-free survival in a patient with stage IVB uterine carcinosarcoma
- Carcinosarcoma of Bladder: Report of a Case
- Validation of Traditional Prognosis Scoring Systems and Skeletal Oncology Research Group Nomogram for Predicting Survival of Spinal Metastasis Patients Undergoing Surgery
- Development and internal validation of a nomogram for predicting outcomes in children with traumatic subdural hematoma