Intest Res.
2023 Apr;21(2):205-215. 10.5217/ir.2021.00164.
Performing colonoscopy before steroid induction is associated with shorter steroid use in patients with ulcerative colitis
- Affiliations
-
- 1Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Japan
- 2Japan Medical Office, Takeda Pharmaceutical Company Limited, Tokyo, Japan
- KMID: 2541893
- DOI: http://doi.org/10.5217/ir.2021.00164
Abstract
- Background/Aims
Risks of long-term steroid use in patients with ulcerative colitis (UC) outweigh the benefits, thus dosing should be tapered once a response is achieved. Colonoscopy is a key technique for assessing disease severity and optimizing treatment involving steroids. This retrospective longitudinal cohort study of patients with UC explored factors associated with the duration of systemic steroid use.
Methods
The Japan Medical Data Center database, an employer-based insurance claims database, was used to select individuals initiating prednisolone, with a prescription issued between January 1, 2010, and January 31, 2018. The study included adults with a confirmed diagnosis of UC, who had received ≥1 year of continuous treatment with 5-aminosalicylic acid, biologics, or thiopurine. Factors associated with prednisolone duration were assessed using a multivariate regression model.
Results
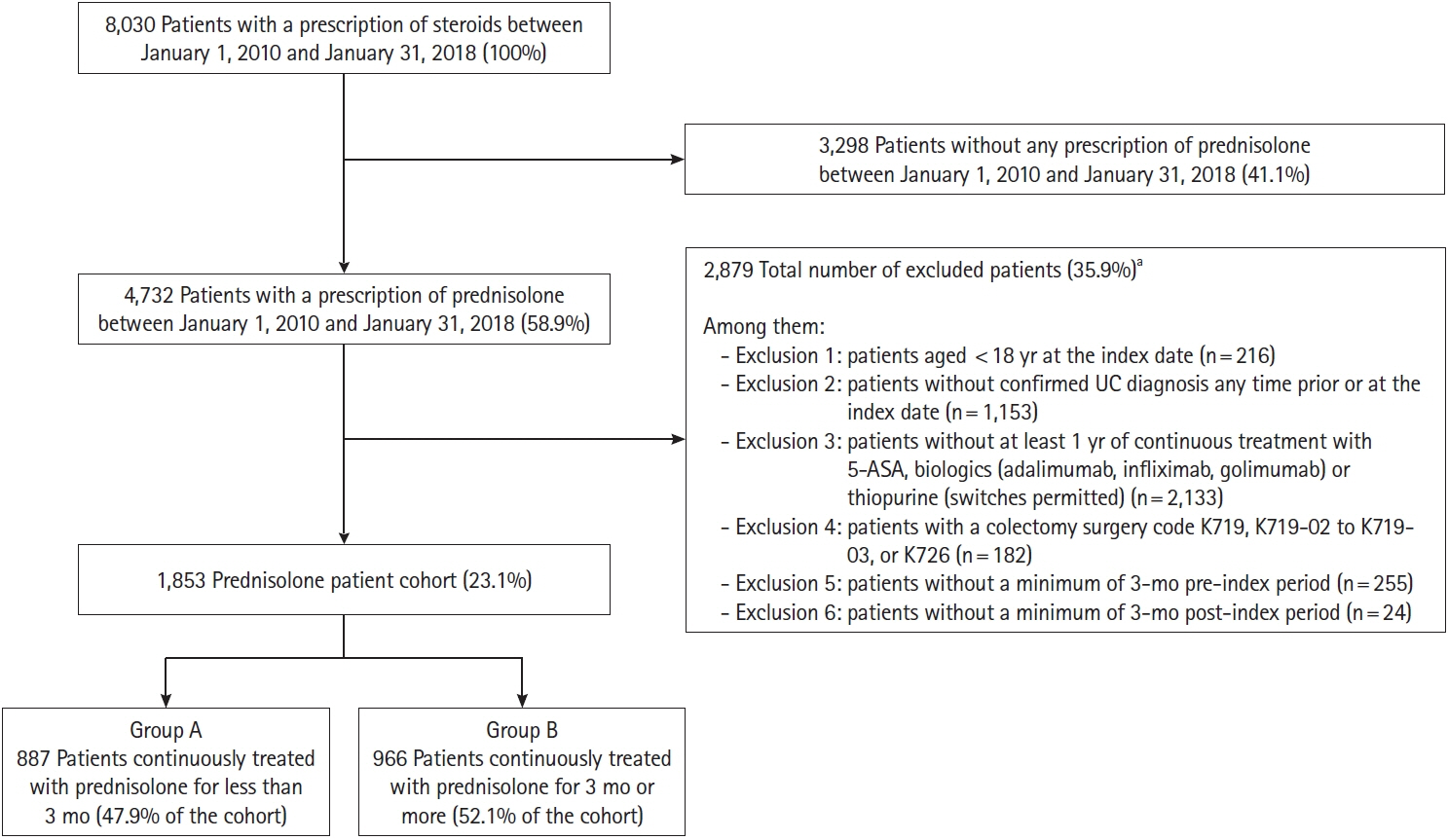
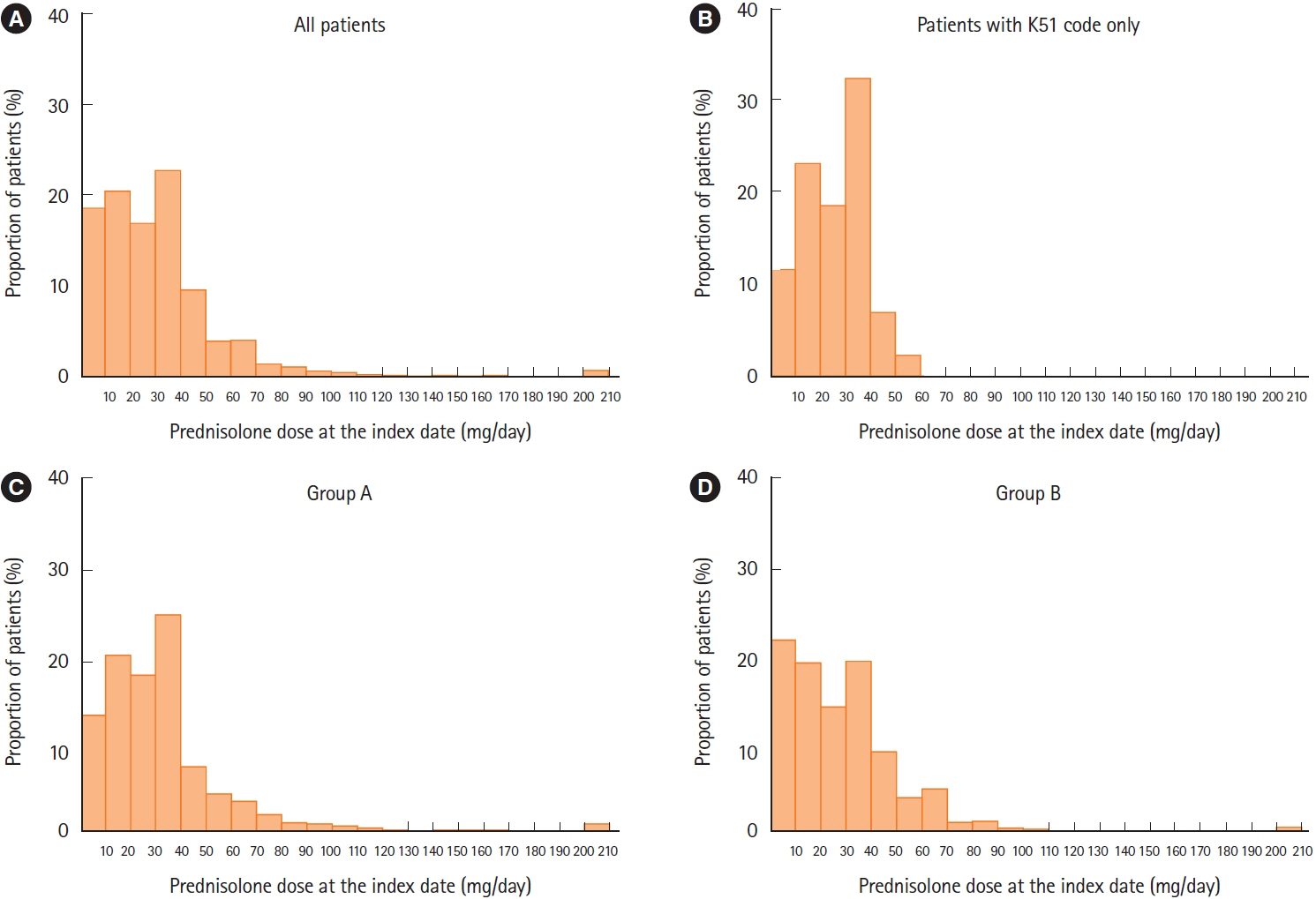
Median duration of prednisolone treatment was 98 days, and colonoscopy was performed ≤1 month before or at the first prescription of prednisolone (index date) in 32.8% of patients (607/1,853). Shorter durations of prednisolone treatment were associated with colonoscopy ≤1 month before or at the index date and higher prednisolone dose at index date, with incidence rate ratios (IRRs) of 0.776 (95% confidence interval [CI], 0.682–0.884; P<0.001) and 0.998 (95% CI, 0.996–1.000; P=0.018), respectively. Charlson Comorbidity Index scores of 1 and ≥2 predicted longer prednisolone treatment (IRR, 1.332; 95% CI, 1.174–1.511; P<0.001 and IRR, 1.599; 95% CI, 1.357–1.885; P<0.001, respectively).
Conclusions
Performing colonoscopy before or at the time of initiating steroid was associated with a shorter duration of steroid use in patients with UC.
Figure
Reference
-
1. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.
Article2. Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020; 6:74.
Article3. Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021; 56:489–526.
Article4. Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol. 2009; 44:659–665.
Article5. Ohfuji S. Epidemiology of ulcerative colitis in Japan. In : Washio M, Kobashi G, editors. Epidemiological studies of specified rare and intractable disease. Singapore: Springer Singapore;2019. p. 117–131.6. Morita N, Toki S, Hirohashi T, et al. Incidence and prevalence of inflammatory bowel disease in Japan: nationwide epidemiological survey during the year 1991. J Gastroenterol. 1995; 30 Suppl 8:1–4.7. Murakami Y, Nishiwaki Y, Oba MS, et al. Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2014: an analysis of a nationwide survey. J Gastroenterol. 2019; 54:1070–1077.
Article8. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017; 389:1756–1770.
Article9. Allez M, Lémann M. Role of endoscopy in predicting the disease course in inflammatory bowel disease. World J Gastroenterol. 2010; 16:2626–2632.
Article10. Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996; 38:905–910.
Article11. Goetz M. Endoscopic surveillance in inflammatory bowel disease. Visc Med. 2018; 34:66–71.
Article12. Spiceland CM, Lodhia N. Endoscopy in inflammatory bowel disease: role in diagnosis, management, and treatment. World J Gastroenterol. 2018; 24:4014–4020.
Article13. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017; 11:769–784.
Article14. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019; 114:384–413.
Article15. Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001; 121:255–260.
Article16. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012; 380:1606–1619.
Article17. Waljee AK, Wiitala WL, Govani S, et al. Corticosteroid use and complications in a US inflammatory bowel disease cohort. PLoS One. 2016; 11:e0158017.
Article18. Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006; 55:47–53.
Article19. Chebli LA, Chaves LD, Pimentel FF, et al. Azathioprine maintains long-term steroid-free remission through 3 years in patients with steroid-dependent ulcerative colitis. Inflamm Bowel Dis. 2010; 16:613–619.
Article20. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476.
Article21. Chhaya V, Saxena S, Cecil E, et al. Steroid dependency and trends in prescribing for inflammatory bowel disease: a 20-year national population-based study. Aliment Pharmacol Ther. 2016; 44:482–494.
Article22. Matsuoka K, Igarashi A, Sato N, et al. Trends in corticosteroid prescriptions for ulcerative colitis and factors associated with long-term corticosteroid use: analysis using Japanese claims data from 2006 to 2016. J Crohns Colitis. 2021; 15:358–366.
Article23. Corte C, Fernandopulle N, Catuneanu AM, et al. Association between the ulcerative colitis endoscopic index of severity (UCEIS) and outcomes in acute severe ulcerative colitis. J Crohns Colitis. 2015; 9:376–381.
Article24. Limketkai BN, Singh S, Jairath V, Sandborn WJ, Dulai PS. US practice patterns and impact of monitoring for mucosal inflammation after biologic initiation in inflammatory bowel disease. Inflamm Bowel Dis. 2019; 25:1828–1837.
Article25. Ananthakrishnan AN, Cagan A, Cai T, et al. Colonoscopy is associated with a reduced risk for colon cancer and mortality in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2015; 13:322–329.
Article26. Selinger CP, Parkes GC, Bassi A, et al. A multi-centre audit of excess steroid use in 1176 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017; 46:964–973.
Article27. Selinger CP, Parkes GC, Bassi A, et al. Assessment of steroid use as a key performance indicator in inflammatory bowel disease: analysis of data from 2385 UK patients. Aliment Pharmacol Ther. 2019; 50:1009–1018.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multi-session fecal microbiota transplantation using colonoscopy has favorable outcomes for the treatment of steroid-dependent ulcerative colitis
- Combined eosinophilic gastroenteritis and ulcerative colitis successfully treated by vedolizumab: a case report
- Treatment of Steroid Refractory Ulcerative Colitis
- Advances in ulcerative colitis therapy
- Steroid-resistant Ulcerative Colitis in Acute Exacervation Phase Remitted by Cyclosporine and Azathioprine: A case report