Ann Rehabil Med.
2023 Apr;47(2):79-88. 10.5535/arm.23013.
Reliability and Validity of the Korean Version of the Duchenne Muscular Dystrophy Functional Ability Self-Assessment Tool
- Affiliations
-
- 1Department of Rehabilitation Medicine, Seoul National University Hospital, Seoul, Korea
- KMID: 2541841
- DOI: http://doi.org/10.5535/arm.23013
Abstract
Objective
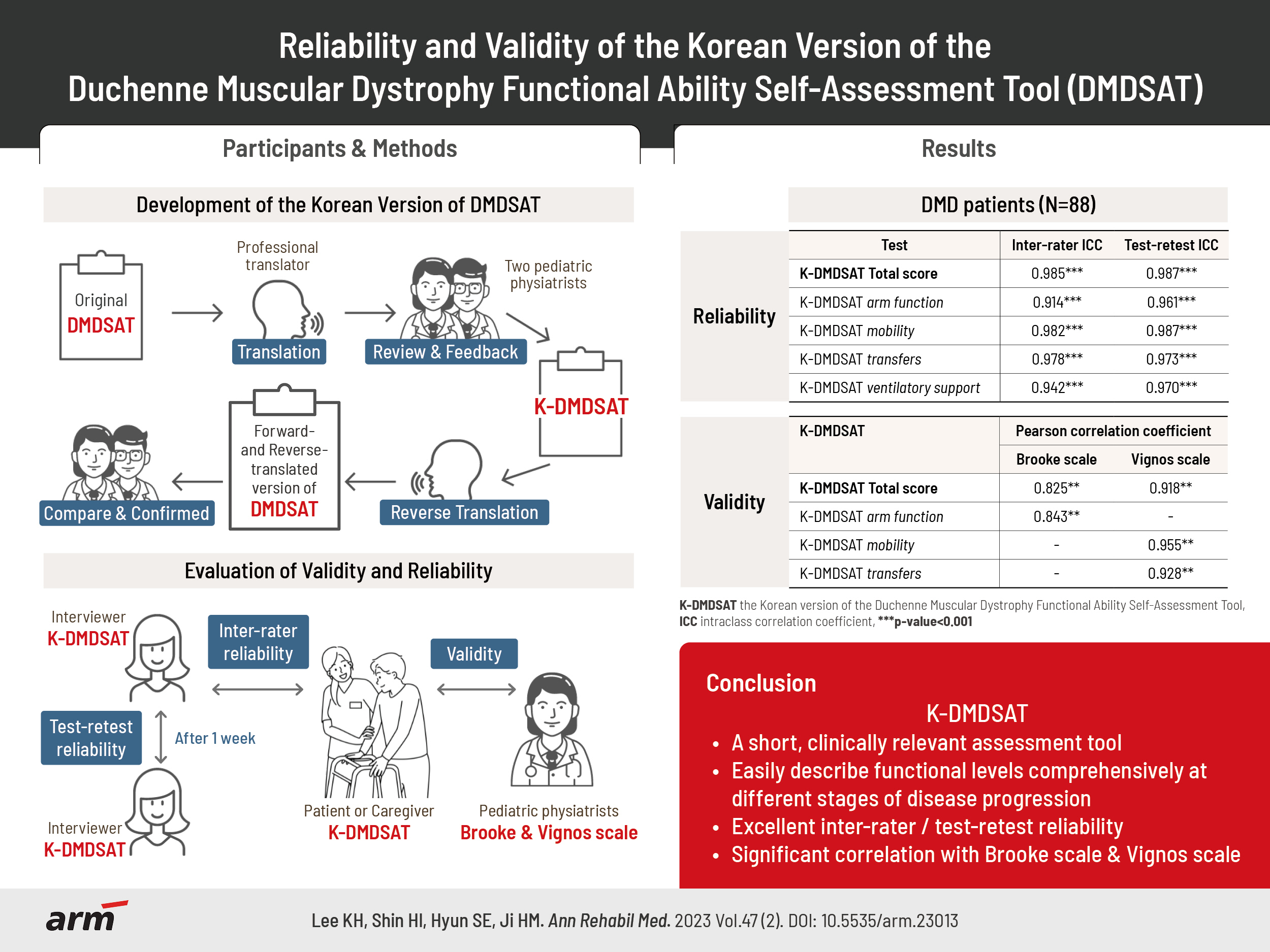
To systematically translate the Duchenne muscular dystrophy Functional Ability Self-Assessment Tool (DMDSAT) into Korean and verify the reliability and validity of the Korean version (K-DMDSAT).
Methods
The original DMDSAT was translated into Korean by two translators and two pediatric physiatrists. A total of 88 patients with genetically confirmed Duchenne muscular dystrophy (DMD) participated in the study. They were evaluated using the K-DMDSAT once as a self-assessment and once by an interviewer. The interviewer evaluated the K-DMDSAT again 1 week later using a test-retest approach. The intraclass correlation coefficient (ICC) was used to verify the interrater and test-retest reliabilities. Pearson correlation analysis between the K-DMDSAT and the Brooke or Vignos scales were used to assess validity.
Results
The total score and all domains of the K-DMDSAT showed excellent interrater and test-retest reliability, with an ICC for total scores of 0.985 and 0.987, respectively. All domains had an ICC >0.90. From the Pearson correlation analysis, the total K-DMDSAT score was significantly correlated with the Vignos and Brooke scales (r=0.918 and 0.825, respectively; p<0.001), and each domain of K-DMDSAT showed significant correlation with either the Vignos or Brooke scales.
Conclusion
DMDSAT was systematically translated into Korean, and K-DMDSAT was verified to have excellent reliability and validity. K-DMDSAT can help clinicians easily describe and categorize various functional aspects of patients with DMD through the entire disease progression.
Keyword
Figure
Cited by 1 articles
-
Muscle Pathology Associated With Cardiac Function in Duchenne Muscular Dystrophy
Jin A Yoon, Heirim Lee, In Sook Lee, You Seon Song, Byeong-Ju Lee, Soo-Yeon Kim, Yong Beom Shin
Ann Rehabil Med. 2024;48(6):405-412. doi: 10.5535/arm.240006.
Reference
-
1. Mah JK, Korngut L, Dykeman J, Day L, Pringsheim T, Jette N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2014; 24:482–91.
Article2. Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987; 51:919–28.
Article3. Mah JK. An overview of recent therapeutics advances for Duchenne muscular dystrophy. Methods Mol Biol. 2018; 1687:3–17.
Article4. Kim A, Park M, Shin HI. Pain characteristics among individuals with Duchenne muscular dystrophy according to their clinical stage. BMC Musculoskelet Disord. 2022; 23:536.
Article5. Canavese F, Sussman MD. Strategies of hip management in neuromuscular disorders: Duchenne Muscular Dystrophy, Spinal Muscular Atrophy, CharcotMarie-Tooth Disease and Arthrogryposis Multiplex Congenita. Hip Int. 2009; 19 Suppl 6:S46–52.
Article6. Mayer OH, Finkel RS, Rummey C, Benton MJ, Glanzman AM, Flickinger J, et al. Characterization of pulmonary function in Duchenne Muscular Dystrophy. Pediatr Pulmonol. 2015; 50:487–94.
Article7. Buddhe S, Cripe L, Friedland-Little J, Kertesz N, Eghtesady P, Finder J, et al. Cardiac management of the patient with Duchenne Muscular Dystrophy. Pediatrics. 2018; 142(Suppl 2):S72–81.
Article8. Landfeldt E, Mayhew A, Eagle M, Lindgren P, Bell CF, Guglieri M, et al. Development and psychometric analysis of the Duchenne muscular dystrophy Functional Ability Self-Assessment Tool (DMDSAT). Neuromuscul Disord 2015;25:937-44. Erratum in: Neuromuscul Disord. 2016; 26:329.
Article9. El-Aloul B, Speechley KN, Wei Y, Wilk P, Campbell C. Fatigue in young people with Duchenne muscular dystrophy. Dev Med Child Neurol. 2020; 62:245–51.
Article10. Arteaga D, Donnelly T, Crum K, Markham L, Killian M, Burnette WB, et al. Assessing physical activity using accelerometers in youth with Duchenne muscular dystrophy. J Neuromuscul Dis. 2020; 7:331–42.
Article11. Fujii T, Takeshita E, Iwata Y, Yajima H, Nozaki F, Mori M, et al. Cumulative jerk as an outcome measure in nonambulatory Duchenne muscular dystrophy. Brain Dev. 2019; 41:796–802.
Article12. Landfeldt E, Lindgren P, Bell CF, Guglieri M, Straub V, Lochmüller H, et al. Quantifying the burden of caregiving in Duchenne muscular dystrophy. J Neurol. 2016; 263:906–15.
Article13. Landfeldt E, Lindgren P, Bell CF, Schmitt C, Guglieri M, Straub V, et al. The burden of Duchenne muscular dystrophy: an international, cross-sectional study. Neurology. 2014; 83:529–36.
Article14. Landfeldt E, Alfredsson L, Straub V, Lochmüller H, Bushby K, Lindgren P. Economic evaluation in Duchenne muscular dystrophy: model frameworks for cost-effectiveness analysis. Pharmacoeconomics. 2017; 35:249–58.
Article15. Salari N, Fatahi B, Valipour E, Kazeminia M, Fatahian R, Kiaei A, et al. Global prevalence of Duchenne and Becker muscular dystrophy: a systematic review and meta-analysis. J Orthop Surg Res. 2022; 17:96.
Article16. Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve. 1981; 4:186–97.
Article17. Vignos PJ Jr, Archibald KC. Maintenance of ambulation in childhood muscular dystrophy. J Chronic Dis. 1960; 12:273–90.
Article18. Bushby K, Connor E. Clinical outcome measures for trials in Duchenne muscular dystrophy: report from International Working Group meetings. Clin Investig (Lond). 2011; 1:1217–35.
Article19. Mazzone ES, Messina S, Vasco G, Main M, Eagle M, D’Amico A, et al. Reliability of the North Star Ambulatory Assessment in a multicentric setting. Neuromuscul Disord. 2009; 19:458–61.
Article20. Bérard C, Payan C, Hodgkinson I, Fermanian J; MFM Collaborative Study Group. A motor function measure for neuromuscular diseases. Construction and validation study. Neuromuscul Disord. 2005; 15:463–70.21. Kim J, Jung IY, Kim SJ, Lee JY, Park SK, Shin HI, et al. A new functional scale and ambulatory functional classification of Duchenne muscular dystrophy: scale development and preliminary analyses of reliability and validity. Ann Rehabil Med. 2018; 42:690–701.
Article22. Mayhew AG, Eagle M, Steffenson B. S.P.6 Exploratory Rasch analysis of the EK2 scale used in a population of Duchenne muscular dystrophy (DMD). Neuromuscul Disord. 2012; 22:877.
Article23. Huang M, Chen T, Wang Y, Zhou C, Cao J, Lu X, et al. Chronic pain, psychological distress, and quality of life in males with Duchenne muscular dystrophy. Dev Med Child Neurol. 2023; 65:640–54.
Article24. MacKintosh EW, Chen ML, Benditt JO. Lifetime care of Duchenne muscular dystrophy. Sleep Med Clin. 2020; 15:485–95.
Article25. Fiorentino G, Annunziata A, Cauteruccio R, Frega GS, Esquinas A. Mouthpiece ventilation in Duchenne muscular dystrophy: a rescue strategy for noncompliant patients. J Bras Pneumol. 2016; 42:453–6.
Article26. Hurvitz MS, Bhattacharjee R, Lesser DJ, Skalsky AJ, Orr JE. Determinants of usage and nonadherence to noninvasive ventilation in children and adults with Duchenne muscular dystrophy. J Clin Sleep Med. 2021; 17:1973–80.
Article27. Gomez-Merino E, Bach JR. Duchenne muscular dystrophy: prolongation of life by noninvasive ventilation and mechanically assisted coughing. Am J Phys Med Rehabil. 2002; 81:411–5.28. Landfeldt E, Thompson R, Sejersen T, McMillan HJ, Kirschner J, Lochmüller H. Life expectancy at birth in Duchenne muscular dystrophy: a systematic review and meta-analysis. Eur J Epidemiol. 2020; 35:643–53.
Article29. Ishikawa Y, Miura T, Ishikawa Y, Aoyagi T, Ogata H, Hamada S, et al. Duchenne muscular dystrophy: survival by cardio-respiratory interventions. Neuromuscul Disord. 2011; 21:47–51.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correction: Reliability and Validity of the Korean Version of the Duchenne Muscular Dystrophy Functional Ability Self-Assessment Tool
- A clinical study on Duchenne muscular dystrophy
- Clinical Implications of Pulmonary Function Test and Maximum Static Pressure in Duchenne Muscular Dystrophy
- Duchenne Muscular Dystrophy Complicated With Dilated Cardiomyopathy and Cerebral Infarction
- A Clinical Study on Duchenne Muscular Dystrophy in Childhood