Nutr Res Pract.
2023 Apr;17(2):192-205. 10.4162/nrp.2023.17.2.192.
The effect of curcumin on blood pressure and cognitive impairment in spontaneously hypertensive rats
- Affiliations
-
- 1Department of Food Science and Nutrition, Dankook University, Cheonan 31116, Korea
- 2Department of Food Science and Nutrition, Natural Nutraceuticals Industrialization Research Center, Dankook University, Cheonan 31116, Korea
- KMID: 2541358
- DOI: http://doi.org/10.4162/nrp.2023.17.2.192
Abstract
- BACKGROUND/OBJECTIVES
It is known that the renin-angiotensin system (RAS) in the brain could regulate cognitive functions as well as blood pressure. Inhibition of RAS for the improvement of cognitive function may be a new strategy, but studies so far have mostly reported on the effects of RAS inhibition by drugs, and there is no research on cognitive improvement through RAS inhibition of food ingredients. Therefore, this study investigated the effect of curcumin on blood pressure and cognitive function and its related mechanism in spontaneously hypertensive rat/Izm (SHR/Izm).
MATERIALS/METHODS
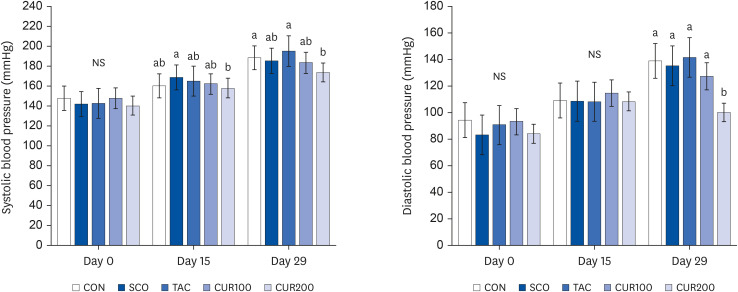
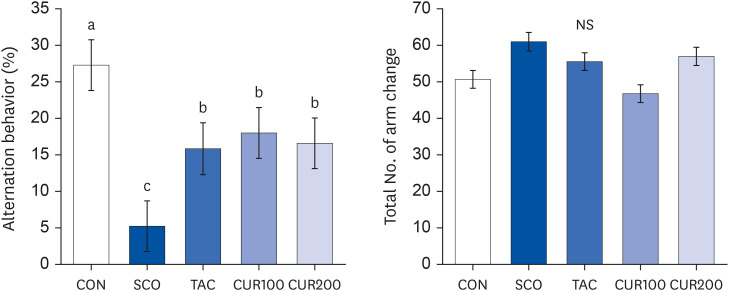
Six-week-old SHR/Izm rats were divided into 5 groups: control group (CON), scopolamine group (SCO, drug for inducing cognitive deficits), positive control (SCO and tacrine [TAC]), curcumin 100 group (CUR100, SCO + Cur 100 mg/kg), and curcumin 200 group (CUR200, SCO + Cur 200 mg/kg). Changes in blood pressure, RAS, cholinergic system, and cognitive function were compared before and after cognitive impairment.
RESULTS
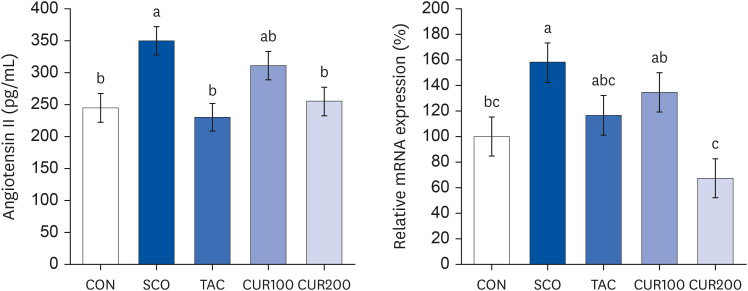
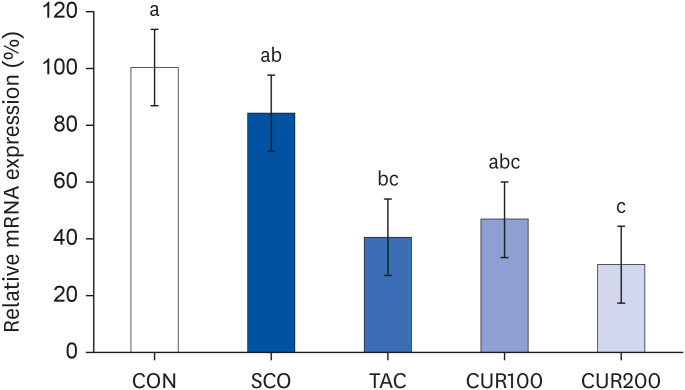
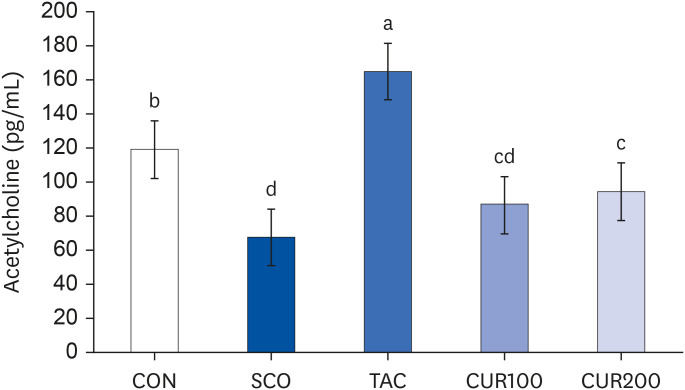
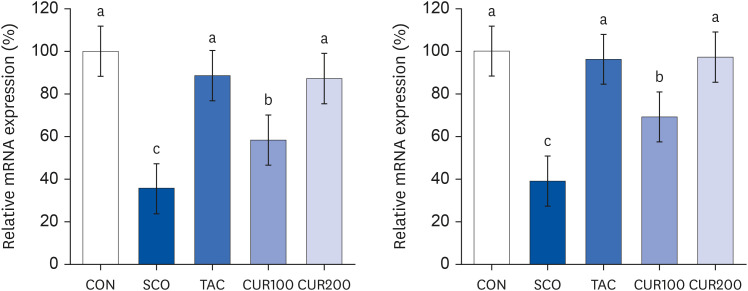
The SCO group showed increased blood pressure and significantly reduced cognitive function based on the y-maze and passive avoidance test. Curcumin treatments significantly improved blood pressure and cognitive function compared with the SCO group. In both the CUR100 and CUR200 groups, the mRNA expressions of angiotensin-converting enzyme (ACE) and angiotensin II receptor type1 (AT1), as well as the concentrations of angiotensin II (Ang II) in brain tissue were significantly decreased. The mRNA expression of the muscarinic acetylcholine receptors (mAChRs) and acetylcholine (ACh) content was significantly increased, compared with the SCO group.
CONCLUSIONS
The administration of curcumin improved blood pressure and cognitive function in SCO-induced hypertensive mice, indicating that the cholinergic system was improved by suppressing RAS and AT1 receptor expression and increasing the mAChR expression.
Figure
Reference
-
1. Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension. 2016; 68:e67–e94. PMID: 27977393.2. Cherubini A, Lowenthal DT, Paran E, Mecocci P, Williams LS, Senin U. Hypertension and cognitive function in the elderly. Dis Mon. 2010; 56:106–147. PMID: 20189499.3. Forte G, Casagrande M. Effects of blood pressure on cognitive performance in aging: a systematic review. Brain Sci. 2020; 10:919. PMID: 33261205.4. de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012; 2012:367516. PMID: 23243502.5. Sunagawa Y, Morimoto T, Wada H, Takaya T, Katanasaka Y, Kawamura T, Yanagi S, Marui A, Sakata R, Shimatsu A, et al. A natural p300-specific histone acetyltransferase inhibitor, curcumin, in addition to angiotensin-converting enzyme inhibitor, exerts beneficial effects on left ventricular systolic function after myocardial infarction in rats. Circ J. 2011; 75:2151–2159. PMID: 21737953.6. Yamada K, Horita T, Takayama M, Takahashi S, Takaba K, Nagata Y, Suzuki N, Kanda T. Effect of a centrally active angiotensin converting enzyme inhibitor, perindopril, on cognitive performance in chronic cerebral hypo-perfusion rats. Brain Res. 2011; 1421:110–120. PMID: 21981801.7. Kumaran D, Udayabanu M, Kumar M, Aneja R, Katyal A. Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience. 2008; 155:626–639. PMID: 18621107.8. Farag E, Sessler DI, Ebrahim Z, Kurz A, Morgan J, Ahuja S, Maheshwari K, John Doyle D. The renin angiotensin system and the brain: New developments. J Clin Neurosci. 2017; 46:1–8. PMID: 28890045.9. Takeda S, Sato N, Ogihara T, Morishita R. The renin-angiotensin system, hypertension and cognitive dysfunction in Alzheimer’s disease: new therapeutic potential. Front Biosci. 2008; 13:2253–2265. PMID: 17981707.10. Wright JW, Harding JW. Contributions by the brain renin-angiotensin system to memory, cognition, and Alzheimer’s disease. J Alzheimers Dis. 2019; 67:469–480. PMID: 30664507.11. Tota S, Hanif K, Kamat PK, Najmi AK, Nath C. Role of central angiotensin receptors in scopolamine-induced impairment in memory, cerebral blood flow, and cholinergic function. Psychopharmacology (Berl). 2012; 222:185–202. PMID: 22362194.12. Sabbatini M, Catalani A, Consoli C, Marletta N, Tomassoni D, Avola R. The hippocampus in spontaneously hypertensive rats: an animal model of vascular dementia? Mech Ageing Dev. 2002; 123:547–559. PMID: 11796140.13. Papadopoulos P, Tong XK, Imboden H, Hamel E. Losartan improves cerebrovascular function in a mouse model of Alzheimer’s disease with combined overproduction of amyloid-β and transforming growth factor-β1. J Cereb Blood Flow Metab. 2017; 37:1959–1970. PMID: 27389178.14. Barnes JM, Barnes NM, Costall B, Horovitz ZP, Naylor RJ. Angiotensin II inhibits the release of [3H]acetylcholine from rat entorhinal cortex in vitro. Brain Res. 1989; 491:136–143. PMID: 2765877.15. Ahmed HA, Ishrat T, Pillai B, Fouda AY, Sayed MA, Eldahshan W, Waller JL, Ergul A, Fagan SC. RAS modulation prevents progressive cognitive impairment after experimental stroke: a randomized, blinded preclinical trial. J Neuroinflammation. 2018; 15:229. PMID: 30103772.16. Royea J, Lacalle-Aurioles M, Trigiani LJ, Fermigier A, Hamel E. AT2R’s (Angiotensin II Type 2 Receptor’s) tole in cognitive and cerebrovascular deficits in a mouse model of Alzheimer disease. Hypertension. 2020; 75:1464–1474. PMID: 32362228.17. Jeong EJ, Lee KY, Kim SH, Sung SH, Kim YC. Cognitive-enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine-treated mice. Eur J Pharmacol. 2008; 588:78–84. PMID: 18462717.18. Davies NM, Kehoe PG, Ben-Shlomo Y, Martin RM. Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J Alzheimers Dis. 2011; 26:699–708. PMID: 21709373.19. Mogi M, Horiuchi M. Effect of angiotensin II type 2 receptor on stroke, cognitive impairment and neurodegenerative diseases. Geriatr Gerontol Int. 2013; 13:13–18. PMID: 22726823.20. Savaskan E. The role of the brain renin-angiotensin system in neurodegenerative disorders. Curr Alzheimer Res. 2005; 2:29–35. PMID: 15977987.21. Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin. J Med Chem. 2017; 60:1620–1637. PMID: 28074653.22. Awasthi H, Tota S, Hanif K, Nath C, Shukla R. Protective effect of curcumin against intracerebral streptozotocin induced impairment in memory and cerebral blood flow. Life Sci. 2010; 86:87–94. PMID: 19925811.23. Abd Allah ES, Gomaa AM. Effects of curcumin and captopril on the functions of kidney and nerve in streptozotocin-induced diabetic rats: role of angiotensin converting enzyme 1. Appl Physiol Nutr Metab. 2015; 40:1061–1067. PMID: 26398443.24. Fan X, Zhang C, Liu DB, Yan J, Liang HP. The clinical applications of curcumin: current state and the future. Curr Pharm Des. 2013; 19:2011–2031. PMID: 23116310.25. Greish SM, Abdel-Hady Z, Mohammed SS, Abdel-Hamed AR, Masoud RE, Eltamany DA, Abogresha NM. Protective potential of curcumin in L-NAME-induced hypertensive rat model: AT1R, mitochondrial DNA synergy. Int J Physiol Pathophysiol Pharmacol. 2020; 12:134–146. PMID: 33224436.26. Sarker MR, Franks SF. Efficacy of curcumin for age-associated cognitive decline: a narrative review of preclinical and clinical studies. Geroscience. 2018; 40:73–95. PMID: 29679204.27. Yu SY, Zhang M, Luo J, Zhang L, Shao Y, Li G. Curcumin ameliorates memory deficits via neuronal nitric oxide synthase in aged mice. Prog Neuropsychopharmacol Biol Psychiatry. 2013; 45:47–53. PMID: 23665290.28. Bassani TB, Turnes JM, Moura EL, Bonato JM, Cóppola-Segovia V, Zanata SM, Oliveira RM, Vital MA. Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behav Brain Res. 2017; 335:41–54. PMID: 28801114.29. Lee J, Kim YS, Kim E, Kim Y, Kim Y. Curcumin and hesperetin attenuate D-galactose-induced brain senescence in vitro and in vivo . Nutr Res Pract. 2020; 14:438–452. PMID: 33029285.30. Kim HR, Kim WK, Ha AW. Effects of phytochemicals on blood pressure and neuroprotection mediated via brain renin-angiotensin system. Nutrients. 2019; 11:2761. PMID: 31739443.31. Ishinaga Y, Nabika T, Shimada T, Hiraoka J, Nara Y, Yamori Y. Re-evaluation of the SA gene in spontaneously hypertensive and Wistar-Kyoto rats. Clin Exp Pharmacol Physiol. 1997; 24:18–22. PMID: 9043800.32. Kishikawa Y, Kawahara Y, Yamada M, Kaneko F, Kawahara H, Nishi A. The spontaneously hypertensive rat/Izm (SHR/Izm) shows attention deficit/hyperactivity disorder-like behaviors but without impulsive behavior: therapeutic implications of low-dose methylphenidate. Behav Brain Res. 2014; 274:235–242. PMID: 25151620.33. Gacar N, Mutlu O, Utkan T, Komsuoglu Celikyurt I, Gocmez SS, Ulak G. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and Morris water maze tests in rats. Pharmacol Biochem Behav. 2011; 99:316–323. PMID: 21624386.34. Tayebati SK, Tomassoni D, Amenta F. Spontaneously hypertensive rat as a model of vascular brain disorder: microanatomy, neurochemistry and behavior. J Neurol Sci. 2012; 322:241–249. PMID: 22726353.35. Chang YM, Ashok Kumar K, Ju DT, Ho TJ, Mahalakshmi B, Lin WT, Day CH, Vijaya Padma V, Liao PH, Huang CY. Dipeptide IF prevents the effects of hypertension-induced Alzheimer’s disease on long-term memory in the cortex of spontaneously hypertensive rats. Environ Toxicol. 2020; 35:570–581. PMID: 31889399.36. Thiratmatrakul S, Yenjai C, Waiwut P, Vajragupta O, Reubroycharoen P, Tohda M, Boonyarat C. Synthesis, biological evaluation and molecular modeling study of novel tacrine-carbazole hybrids as potential multifunctional agents for the treatment of Alzheimer’s disease. Eur J Med Chem. 2014; 75:21–30. PMID: 24508831.37. Rahmati B, Kiasalari Z, Roghani M, Khalili M, Ansari F. Antidepressant and anxiolytic activity of Lavandula officinalis aerial parts hydroalcoholic extract in scopolamine-treated rats. Pharm Biol. 2017; 55:958–965. PMID: 28166686.38. Mugwagwa AT, Gadaga LL, Pote W, Tagwireyi D. Antiamnesic Effects of a hydroethanolic extract of Crinum macowanii on Scopolamine-induced memory impairment in mice. J Neurodegener Dis. 2015; 2015:242505. PMID: 26558135.39. Zhang L, Fang Y, Xu Y, Lian Y, Xie N, Wu T, Zhang H, Sun L, Zhang R, Wang Z. Curcumin improves amyloid β-peptide (1-42) induced a spatial memory deficits through BDNF-ERK signaling pathway. PLoS One. 2015; 10:e0131525. PMID: 26114940.40. SoukhakLari R, Moezi L, Pirsalami F, Ashjazadeh N, Moosavi M. Curcumin ameliorates scopolamine-induced mice memory retrieval deficit and restores hippocampal p-Akt and p-GSK-3β. Eur J Pharmacol. 2018; 841:28–32. PMID: 30321530.41. Kamali Dolatabadi L, Emamghoreishi M, Namavar MR, Badeli Sarkala H. Curcumin effects on memory impairment and restoration of irregular neuronal distribution in the hippocampal CA1 region after global cerebral ischemia in male rats. Basic Clin Neurosci. 2019; 10:527–539. PMID: 32284841.42. Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol. 2016; 14:101–115. PMID: 26813123.43. Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, Wolozin B. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010; 340:b5465. PMID: 20068258.44. Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005; 81(Suppl):243S–255S. PMID: 15640487.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Supplementary Effect of Lentinus Edodes on Serum and Hepatic Lipid Levels in Spontaneously Hypertensive Rat
- Antihypertensive Properties of Dried Radish Leaves Powder in Spontaneously Hypertensive Rats
- Electron Microscopic Study of Enalapril Effect on Left Ventricular Hypertrophy in Spontaneously Hypertensive Rat
- The effect of intrathecal curcumin on mechanical allodynia in rats after L5 spinal nerve ligation
- Effect of Ginseng on the Blood Pressure and Lipid Metabolism, during Development of Hypertension in Spontaneously Hypertensive Rat