J Pathol Transl Med.
2023 Mar;57(2):132-137. 10.4132/jptm.2023.02.07.
Unusual biclonal IgA plasma cell myeloma with aberrant expression of high-risk immunophenotypes: first report of a new diagnostic and clinical challenge
- Affiliations
-
- 1School of Medicine, Ponce Health Sciences University, Ponce, Puerto Rico, USA
- 2Department of Basic Sciences, School of Medicine - Ponce Health Sciences University, Ponce, Puerto Rico, USA
- 3Southern Pathology Services, Inc., Ponce, Puerto Rico, USA
- KMID: 2540500
- DOI: http://doi.org/10.4132/jptm.2023.02.07
Abstract
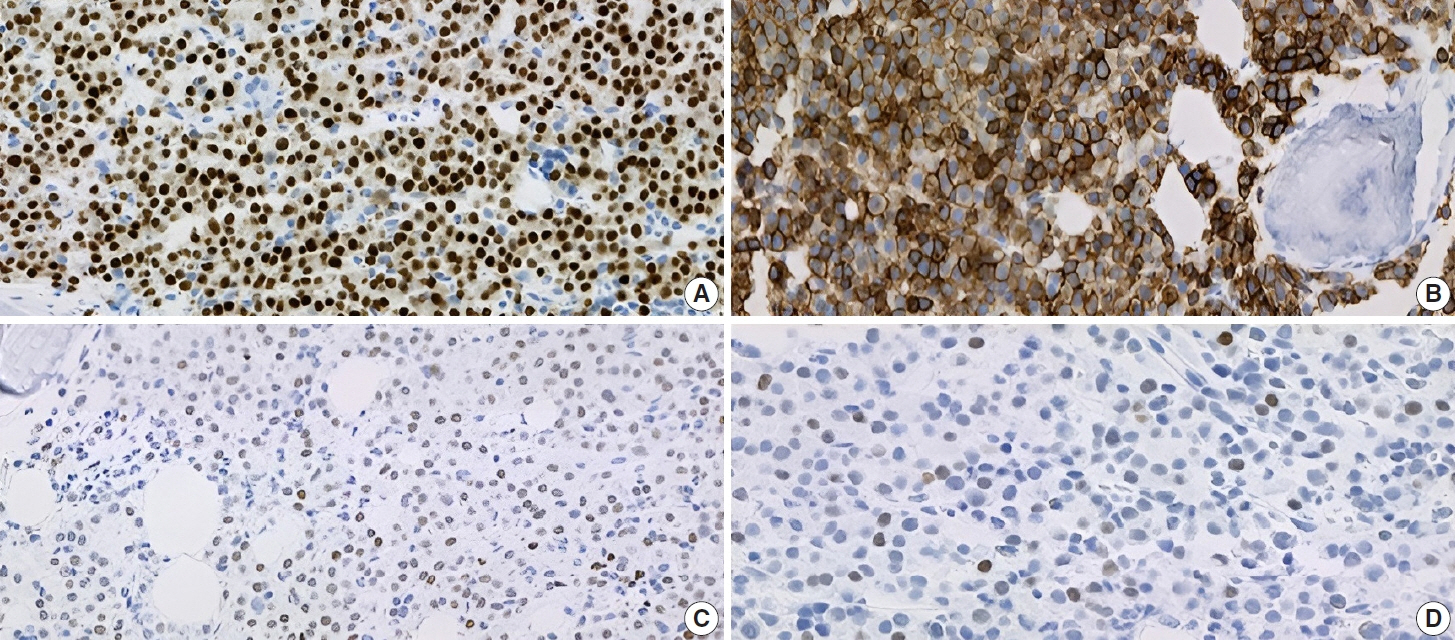
- IgA plasma cell myeloma (PCM) has been linked to molecular abnormalities that confer a higher risk for adverse patient outcomes. However, since IgA PCM only accounts for approximately 20% of all PCM, there are very few reports on high-risk IgA PCM. Moreover, no such reports are found on the more infrequent biclonal IgA PCM. Hence, we present a 65-year-old Puerto Rican female with acute abdominal pain, concomitant hypercalcemia, and acute renal failure. Protein electrophoresis with immunofixation found high IgA levels and detected a biclonal IgA gammopathy with kappa specificity. Histomorphologically, bone marrow showed numerous abnormal plasma cells (32%) replacing over 50% of the marrow stroma. Immunophenotyping analysis detected CD45-negative plasma cells aberrantly expressing CD33, CD43, OCT-2, and c-MYC. Chromosomal analysis revealed multiple abnormalities including the gain of chromosome 1q. Thus, we report on an unusual biclonal IgA PCM and the importance of timely diagnosing aggressive plasma cell neoplasms.
Figure
Reference
-
References
1. Habermehl GK, Nakashima MO, Cotta CV. IgA plasma cell neoplasms are characterized by poorer long-term survival and increased genomic complexity compared to IgG neoplasms. Ann Diagn Pathol. 2020; 44:151449.2. McKenna RW, Kroft SH, Linden MA. Plasma cell neoplasms. In : Jaffe ES, Arber DA, Campo E, Harris NL, Quintanilla-Martinez L, editors. Hematopathology. 2nd. Philadelphia: Elsevier;2017. p. 473–506.3. Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020; 95:548–67.4. Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for Multiple Myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015; 33:2863–9.5. Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005; 90:1128–32.6. Willrich MA, Katzmann JA. Laboratory testing requirements for diagnosis and follow-up of multiple myeloma and related plasma cell dyscrasias. Clin Chem Lab Med. 2016; 54:907–19.7. Keren DF, Schroeder L. Challenges of measuring monoclonal proteins in serum. Clin Chem Lab Med. 2016; 54:947–61.8. Rossi F, Petrucci MT, Guffanti A, et al. Proposal and validation of prognostic scoring systems for IgG and IgA monoclonal gammopathies of undetermined significance. Clin Cancer Res. 2009; 15:4439–45.9. Cardona-Benavides IJ, de Ramon C, Gutierrez NC. Genetic abnormalities in multiple myeloma: prognostic and therapeutic implications. Cells. 2021; 10:336.10. Annibali O, Marchesi F, Petrucci MT, Tirindelli MC, Avvisati G. Relapse of IgA lambda multiple myeloma presenting as obstructive jaundice and abdominal pain. Onkologie. 2009; 32:119–21.11. Cerqueira A, Seco T, Paiva D, Martins H, Cotter J. Acute kidney failure: when multiple myeloma doesn’t give additional clues. Cureus. 2020; 12:e7664.12. Suo L, Liu S, Vega I, Thrall M. Extramedullary multiple myeloma involving the liver and periportal lymph node, diagnosed by EUSFNA in a patient with cirrhosis. Diagn Cytopathol. 2020; 48:657–61.13. Yamane F, Ohta R, Sano C. Left lower abdominal pain as an initial symptom of multiple myeloma. Cureus. 2021; 13:e20652.14. Muchtar E, Gertz MA. The colorful landscape of multiple myeloma. Leuk Lymphoma. 2019; 60:2099–100.15. Ning X, Wei Y, Wei X, et al. CD43 expression is an adverse prognostic factor in newly diagnosed multiple myeloma. Blood. 2021; 138(Suppl 1):4753.16. Toman I, Loree J, Klimowicz AC, et al. Expression and prognostic significance of Oct2 and Bob1 in multiple myeloma: implications for targeted therapeutics. Leuk Lymphoma. 2011; 52:659–67.17. Kodali S, Yu H, Cerny J. Assessing MYC expression in multiple myeloma. J Hematol Thrombo Dis. 2015; 3:e119.18. Castaneda-Avila MA, Ortiz-Ortiz KJ, Torres-Cintron CR, Birmann BM, Epstein MM. Trends in cause of death among patients with multiple myeloma in Puerto Rico and the United States SEER population, 1987-2013. Int J Cancer. 2020; 146:35–43.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Multiple Myeloma with Biclonal Gammopathy
- A Case of Multiple Myeloma with Biclonal (IgG-K and IgA-K) M-proteins
- A Case of Multiple Myeloma with Biclonal Gammopathy
- A Case of Lymphoplasmacytic Lymphoma/Waldenström's Macroglobulinemia with IgM-κ and IgA-λ Biclonal Gammopathy
- Plasma Cell Myeloma: A Report of 2 Cases