Survival Comparison of Incidentally Found versus Clinically Detected Thyroid Cancers: An Analysis of a Nationwide Cohort Study
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Center for Thyroid Cancer, National Cancer Center, Goyang, Korea
- 3Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 4Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea
- 5Integrated Major in Innovative Medical Science, Seoul National University College of Medicine, Seoul, Korea
- 6Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 7Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Korea
- KMID: 2539822
- DOI: http://doi.org/10.3803/EnM.2023.1668
Abstract
- Background
The true benefit of thyroid cancer screening is incompletely understood. This study investigated the impact of ultrasound screening on thyroid cancer outcomes through a comparison with symptomatic thyroid cancer using data from a nationwide cohort study in Korea.
Methods
Cox regression analysis was performed to assess the hazard ratios (HRs) for all-cause and thyroid cancer-specific mortality. Considering the possible bias arising from age, sex, year of thyroid cancer registration, and confounding factors for mortality (including smoking/drinking status, diabetes, and hypertension), all analyses were conducted with stabilized inverse probability of treatment weighting (IPTW) according to the route of detection.
Results
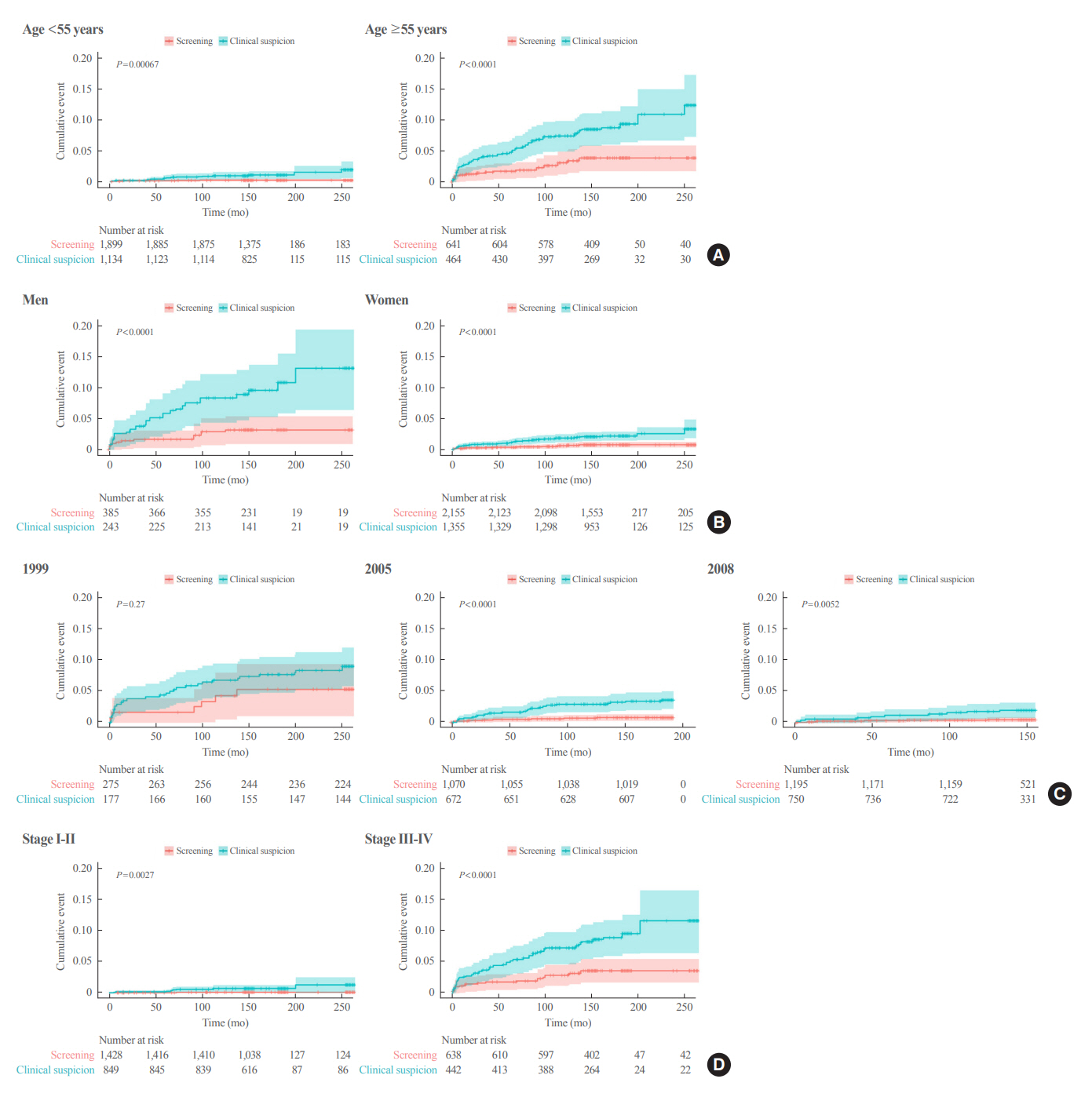
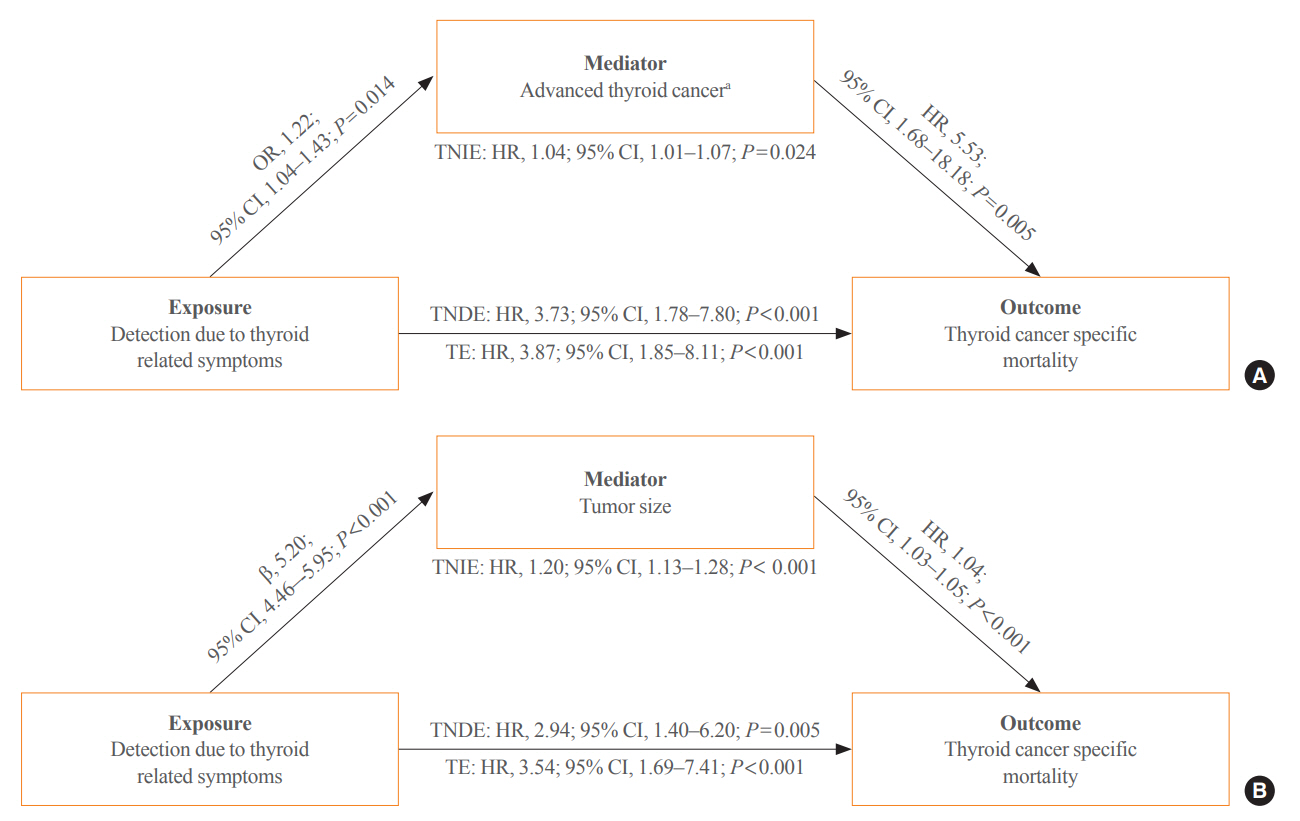
Of 5,796 patients with thyroid cancer, 4,145 were included and 1,651 were excluded due to insufficient data. In comparison with the screening group, the clinical suspicion group was associated with large tumors (17.2±14.6 mm vs. 10.4±7.9 mm), advanced T stage (3–4) (odds ratio [OR], 1.24; 95% confidence interval [CI], 1.09 to 1.41), extrathyroidal extension (OR, 1.16; 95% CI, 1.02 to 1.32), and advanced stage (III–IV) (OR, 1.16; 95% CI, 1.00 to 1.35). In IPTW-adjusted Cox regression analysis, the clinical suspicion group had significantly higher risks of all-cause mortality (HR, 1.43; 95% CI, 1.14 to 1.80) and thyroid cancer-specific mortality (HR, 3.07; 95% CI, 1.77 to 5.29). Mediation analysis showed that the presence of thyroid-specific symptoms was directly associated with a higher risk of cancer-specific mortality. Thyroid-specific symptoms also indirectly affected thyroid cancer-specific mortality, mediated by tumor size and advanced clinicopathologic status.
Conclusion
Our findings provide important evidence for the survival benefit of early detection of thyroid cancer compared to symptomatic thyroid cancer.
Keyword
Figure
Cited by 6 articles
-
Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinol Metab. 2023;38(1):75-77. doi: 10.3803/EnM.2023.105.To Screen or Not to Screen?
Do Joon Park
Endocrinol Metab. 2023;38(1):69-71. doi: 10.3803/EnM.2023.104.The 2017 United States Preventive Services Task Force Recommendation for Thyroid Cancer Screening Is No Longer the Gold Standard
Ka Hee Yi
Endocrinol Metab. 2023;38(1):72-74. doi: 10.3803/EnM.2023.106.Lower Thyroid Cancer Mortality in Patients Detected by Screening: A Meta-Analysis
Shinje Moon, Young Shin Song, Kyong Yeun Jung, Eun Kyung Lee, Young Joo Park
Endocrinol Metab. 2023;38(1):93-103. doi: 10.3803/EnM.2023.1667.Cost-Utility Analysis of Early Detection with Ultrasonography of Differentiated Thyroid Cancer: A Retrospective Study on a Korean Population
Han-Sang Baek, Jeonghoon Ha, Kwangsoon Kim, Ja Seong Bae, Jeong Soo Kim, Sungju Kim, Dong-Jun Lim, Chul-Min Kim
Endocrinol Metab. 2024;39(2):310-323. doi: 10.3803/EnM.2023.1870.Distinct Impacts of Clinicopathological and Mutational Profiles on Long-Term Survival and Recurrence in Medullary Thyroid Carcinoma
Moon Young Oh, Kyong Yeun Jung, Hoonsung Choi, Young Jun Chai, Sun Wook Cho, Su-jin Kim, Kyu Eun Lee, Eun-Jae Chung, Do Joon Park, Young Joo Park, Han-Kwang Yang
Endocrinol Metab. 2024;39(6):877-890. doi: 10.3803/EnM.2024.2027.
Reference
-
1. Brito JP, Al Nofal A, Montori VM, Hay ID, Morris JC. The impact of subclinical disease and mechanism of detection on the rise in thyroid cancer incidence: a population-based study in Olmsted County, Minnesota during 1935 through 2012. Thyroid. 2015; 25:999–1007.
Article2. Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020; 16:17–29.
Article3. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Pineros M, et al. Global cancer observatory: cancer today. Lyon: International Agency for Research on Cancer;2018.4. Kitahara CM, McCullough ML, Franceschi S, Rinaldi S, Wolk A, Neta G, et al. Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies. Thyroid. 2016; 26:306–18.
Article5. Aschebrook-Kilfoy B, DellaValle CT, Purdue M, Kim C, Zhang Y, Sjodin A, et al. Polybrominated diphenyl ethers and thyroid cancer risk in the Prostate, Colorectal, Lung, and Ovarian Cancer Screening Trial cohort. Am J Epidemiol. 2015; 181:883–8.
Article6. Choi YM, Lee J, Kwak MK, Jeon MJ, Kim TY, Hong EG, et al. Recent changes in the incidence of thyroid cancer in Korea between 2005 and 2018: analysis of Korean National Data. Endocrinol Metab (Seoul). 2022; 37:791–9.
Article7. Jung CK, Bae JS, Park YJ. Re-increasing trends in thyroid cancer incidence after a short period of decrease in Korea: reigniting the debate on ultrasound screening. Endocrinol Metab (Seoul). 2022; 37:816–8.
Article8. Li M, Dal Maso L, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020; 8:468–70.
Article9. Lincango-Naranjo E, Solis-Pazmino P, El Kawkgi O, Salazar-Vega J, Garcia C, Ledesma T, et al. Triggers of thyroid cancer diagnosis: a systematic review and meta-analysis. Endocrine. 2021; 72:644–59.
Article10. Chen Z, Mosha SS, Zhang T, Xu M, Li Y, Hu Z, et al. Incidence of microcarcinoma and non-microcarcinoma in ultrasound-found thyroid nodules. BMC Endocr Disord. 2021; 21:38.
Article11. Jun JK, Hwang SY, Hong S, Suh M, Choi KS, Jung KW. Association of screening by thyroid ultrasonography with mortality in thyroid cancer: a case-control study using data from two national surveys. Thyroid. 2020; 30:396–400.
Article12. Chooi JE, Ravindiran A, Balasubramanian SP. The influence of incidental detection of thyroid nodule on thyroid cancer risk and prognosis: a systematic review. Clin Endocrinol (Oxf). 2022; 96:246–54.13. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022; 10:264–72.
Article14. Oh CM, Kong HJ, Kim E, Kim H, Jung KW, Park S, et al. National Epidemiologic Survey of Thyroid cancer (NEST) in Korea. Epidemiol Health. 2018; 40:e2018052.
Article15. Li Y, Yoshida K, Kaufman JS, Mathur MB. A brief primer on conducting regression-based causal mediation analysis. OSF Preprints. 2022; Mar. 28. [Preprint]. https://doi.org/10.31219/osf.io/jath7.
Article16. Krajewska J, Kukulska A, Oczko-Wojciechowska M, Kotecka-Blicharz A, Drosik-Rutowicz K, Haras-Gil M, et al. Early diagnosis of low-risk papillary thyroid cancer results rather in overtreatment than a better survival. Front Endocrinol (Lausanne). 2020; 11:571421.
Article17. Li M, Brito JP, Vaccarella S. Long-term declines of thyroid cancer mortality: an international age-period-cohort analysis. Thyroid. 2020; 30:838–46.
Article18. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017; 317:1338–48.
Article19. Megwalu UC, Moon PK. Thyroid cancer incidence and mortality trends in the United States: 2000-2018. Thyroid. 2022; 32:560–70.
Article20. Jung YS, Oh CM, Kim Y, Jung KW, Ryu J, Won YJ. Long-term survival of patients with thyroid cancer according to the methods of tumor detection: a nationwide cohort study in Korea. PLoS One. 2018; 13:e0194743.
Article21. Bahl M, Sosa JA, Nelson RC, Esclamado RM, Choudhury KR, Hoang JK. Trends in incidentally identified thyroid cancers over a decade: a retrospective analysis of 2,090 surgical patients. World J Surg. 2014; 38:1312–7.
Article22. Choi YJ, Park YL, Koh JH. Prevalence of thyroid cancer at a medical screening center: pathological features of screen-detected thyroid carcinomas. Yonsei Med J. 2008; 49:748–56.
Article23. Chung WY, Chang HS, Kim EK, Park CS. Ultrasonographic mass screening for thyroid carcinoma: a study in women scheduled to undergo a breast examination. Surg Today. 2001; 31:763–7.
Article24. Evranos B, Polat SB, Cuhaci FN, Baser H, Topaloglu O, Kilicarslan A, et al. A cancer of undetermined significance: Incidental thyroid carcinoma. Diagn Cytopathol. 2019; 47:412–6.
Article25. Kim H, Park SY, Jung J, Kim JH, Hahn SY, Shin JH, et al. Improved survival after early detection of asymptomatic distant metastasis in patients with thyroid cancer. Sci Rep. 2019; 9:18745.
Article26. Kim SH, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, et al. Differences in the recurrence and survival of patients with symptomatic and asymptomatic papillary thyroid carcinoma: an observational study of 11,265 person-years of follow-up. Thyroid. 2016; 26:1472–9.
Article27. Zagzag J, Malone MK, Lopresti MA, Ogilvie JB, Patel KN, Heller KS. Method of detection of well-differentiated thyroid cancers in obese and non-obese patients. PLoS One. 2016; 11:e0152768.
Article28. Marina M, Ceda GP, Aldigeri R, Ceresini G. Causes of referral to the first endocrine visit of patients with thyroid carcinoma in a mildly iodine-deficient area. Endocrine. 2017; 57:247–55.
Article29. Pisanu A, Reccia I, Nardello O, Uccheddu A. Risk factors for nodal metastasis and recurrence among patients with papillary thyroid microcarcinoma: differences in clinical relevance between nonincidental and incidental tumors. World J Surg. 2009; 33:460–8.
Article30. Shakil J, Ansari MZ, Brady J, Xu J, Robbins RJ. Lower rates of residual/recurrent disease in patients with incidentally discovered thyroid carcinoma. Endocr Pract. 2017; 23:163–9.
Article31. Kim H, Hwangbo Y, Kong SH, Song YS, Kim MJ, Cho SW, et al. Secular trends for diagnostic motives and environmental risk factors in thyroid cancer using questionnaire survey. Int J Thyroidol. 2017; 10:82–8.
Article32. Palme CE, Waseem Z, Raza SN, Eski S, Walfish P, Freeman JL. Management and outcome of recurrent well-differentiated thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2004; 130:819–24.
Article33. Hu D, Zhou W, Huang Y, Chen S, Zeng W, Wei W, et al. Synergistic effect of clinicopathological factors on mortality risk in patients with differentiated thyroid cancer: an analysis using the SEER database. Surg Oncol. 2020; 34:96–102.
Article34. Robenshtok E, Neeman B, Reches L, Ritter A, Bachar G, Kaminer K, et al. Adverse histological features of differentiated thyroid cancer are commonly found in autopsy studies: implications for treatment guidelines. Thyroid. 2022; 32:37–45.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Screening of Thyroid Cancer and Management of Thyroid Incidentaloma
- Thyroid Hemiagenesis Associated with Micropapillary Thyroid Carcinoma
- Evaluation of Mass Screening for Thyroid Cancer
- A Case of Thyroid Cancer Detected with Pet Scan
- Clinical Characteristics of Incidentally Detected Renal Cell Carcinoma