J Korean Med Sci.
2023 Feb;38(8):e57. 10.3346/jkms.2023.38.e57.
Two Case Reports of Chronic Inflammatory Demyelinating Polyneuropathy After COVID-19 Vaccination
- Affiliations
-
- 1Department of Neurology, Chungnam National University Hospital, Chungnam National University College of Medicine, Daejeon, Korea
- 2Department of Neurology, Chungnam National University Sejong Hospital, Chungnam National University College of Medicine, Sejong, Korea
- KMID: 2539630
- DOI: http://doi.org/10.3346/jkms.2023.38.e57
Abstract
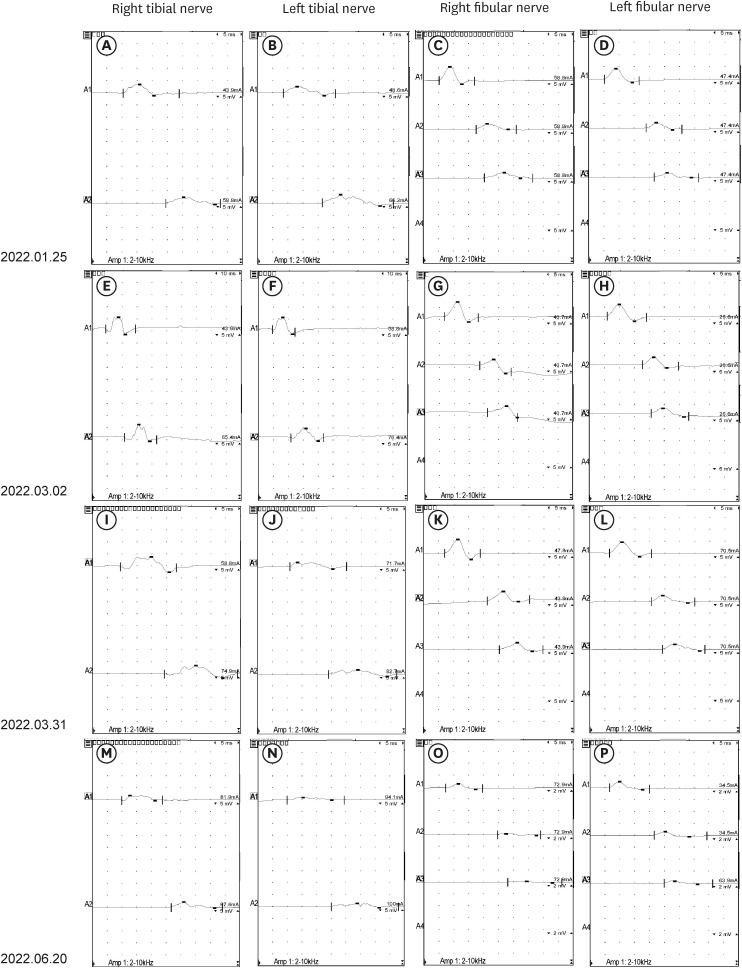
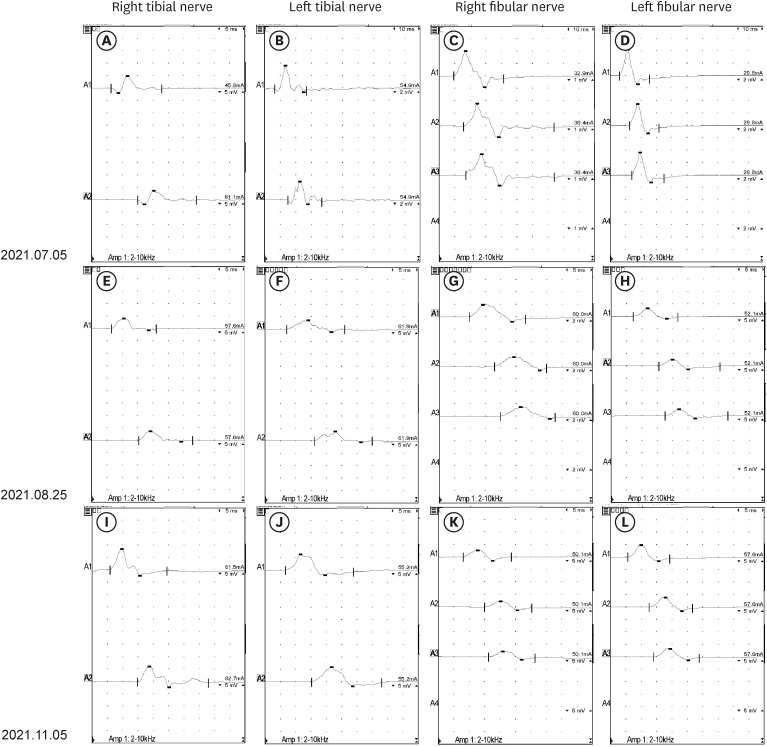
- The occurrence of chronic inflammatory demyelinating polyneuropathy (CIDP) related to coronavirus disease 2019 (COVID-19) has rarely been reported. We describe two patients who were diagnosed with CIDP after COVID-19 vaccination. A 72-year-old man presented with a progressive tingling sensation and weakness below both knees for two weeks. He had been vaccinated against COVID-19 (mRNA-1273 vaccine) a month before the appearance of symptoms. Demyelinating polyneuropathy was observed in the nerve conduction studies (NCS). Intravenous immunoglobulin (IVIg) was administered under the diagnosis of GuillainBarré syndrome (GBS), and his symptoms were improved. However, his symptoms relapsed at 10 weeks from the onset. Oral prednisolone, azathioprine, and IVIg were administered as treatment. The second case was a 50-year-old man who complained of a bilateral leg tingling sensation and gait disturbance lasting four weeks. He had received the Ad26.COV2.S vaccine against COVID-19 five weeks prior. Demyelinating polyneuropathy was observed in the NCS. He was treated with oral prednisolone, azathioprine, and IVIg for CIDP because his symptoms had lasted for more than 12 weeks from the onset. A causal relationship has not been established between COVID-19 vaccination and CIDP; however, CIDP may follow COVID-19 vaccination. As CIDP treatment is different from that for GBS, clinicians should closely monitor patients diagnosed with GBS associated with COVID-19 whether they deteriorate after initial treatment.
Keyword
Figure
Reference
-
1. Van den Bergh PY, Hadden RD, Bouche P, Cornblath DR, Hahn A, Illa I, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. Eur J Neurol. 2010; 17(3):356–363. PMID: 20456730.
Article2. Van den Bergh PY, van Doorn PA, Hadden RD, Avau B, Vankrunkelsven P, Allen JA, et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force-second revision. J Peripher Nerv Syst. 2021; 26(3):242–268. PMID: 34085743.
Article3. Van der Meché FG, Van Doorn PA, Meulstee J, Jennekens FG. GBS-consensus group of the Dutch Neuromuscular Research Support Centre. Diagnostic and classification criteria for the Guillain-Barré syndrome. Eur Neurol. 2001; 45(3):133–139. PMID: 11306855.
Article4. Sriwastava S, Sharma K, Khalid SH, Bhansali S, Shrestha AK, Elkhooly M, et al. COVID-19 vaccination and neurological manifestations: a review of case reports and case series. Brain Sci. 2022; 12(3):407. PMID: 35326363.
Article5. Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2021; 268(4):1133–1170. PMID: 32840686.
Article6. Oh SJ. Clinical Electromyography: Nerve Conduction Studies. Philadelphia, PA, USA: Lippincott Williams & Wilkins;1993.7. Hanson KE, Goddard K, Lewis N, Fireman B, Myers TR, Bakshi N, et al. Incidence of Guillain-Barré syndrome after COVID-19 vaccination in the Vaccine Safety Datalink. JAMA Netw Open. 2022; 5(4):e228879. PMID: 35471572.
Article8. Kim JE, Min YG, Shin JY, Kwon YN, Bae JS, Sung JJ, et al. Guillain-Barré syndrome and variants following COVID-19 vaccination: report of 13 cases. Front Neurol. 2022; 12:820723. PMID: 35153993.
Article9. Leonhard SE, Mandarakas MR, Gondim FA, Bateman K, Ferreira ML, Cornblath DR, et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol. 2019; 15(11):671–683. PMID: 31541214.10. Suri V, Pandey S, Singh J, Jena A. Acute-onset chronic inflammatory demyelinating polyneuropathy after COVID-19 infection and subsequent ChAdOx1 nCoV-19 vaccination. BMJ Case Rep. 2021; 14(10):e245816.11. Abo-Zed A, Pinevich A. Guillain-Barré syndrome, or acute on chronic inflammatory demyelinating polyneuropathy, following Moderna COVID-19 vaccine. Chest. 2021; 160(4):A898.12. de Souza A, Oo WM, Giri P. Inflammatory demyelinating polyneuropathy after the ChAdOx1 nCoV-19 vaccine may follow a chronic course. J Neurol Sci. 2022; 436:120231. PMID: 35313224.
Article13. Pascual-Goñi E, Martín-Aguilar L, Querol L. Autoantibodies in chronic inflammatory demyelinating polyradiculoneuropathy. Curr Opin Neurol. 2019; 32(5):651–657. PMID: 31306213.14. Albert LJ, Inman RD. Molecular mimicry and autoimmunity. N Engl J Med. 1999; 341(27):2068–2074. PMID: 10615080.
Article15. Kim JW, Kim YG, Park YC, Choi S, Lee S, Min HJ, et al. Guillain-Barre syndrome after two COVID-19 vaccinations: two case reports with follow-up electrodiagnostic study. J Korean Med Sci. 2022; 37(7):e58. PMID: 35191234.
Article16. Maramattom BV, Krishnan P, Paul R, Padmanabhan S, Cherukudal Vishnu Nampoothiri S, Syed AA, et al. Guillain-Barré syndrome following ChAdOx1-S/nCoV-19 vaccine. Ann Neurol. 2021; 90(2):312–314. PMID: 34114256.
Article17. Patel SU, Khurram R, Lakhani A, Quirk B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep. 2021; 14(4):e242956.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spectrum of Neurological Complications Following COVID-19 Vaccination in India
- Subacute Inflammatory Demyelinating Polyneuropathy Combined with Optic Neuritis
- Interpretation of Electrodiagnostic Tests in Chronic Inflammatory Demyelinating Polyneuropathy: Classification Using Nerve Conduction Study
- A Review for Vaccination Guidelines in Patients with Central Nervous System Demyelinating Diseases including COVID-19 Vaccination
- Delayed Relaxation (Pseudomyotonia) as the Only Clinical Manifestation of Chronic Inflammatory Demyelinating Polyneuropathy