J Korean Med Sci.
2023 Feb;38(5):e22. 10.3346/jkms.2023.38.e22.
Immunogenicity of SARS-CoV-2 Vaccine in Kidney Transplant Recipients: A Cross-Sectional Study in Korea
- Affiliations
-
- 1Department of Surgery, Ewha Womans University College of Medicine, Seoul, Korea
- 2Advanced Biomedical Research Institute, Ewha Womans University Seoul Hospital, Seoul, Korea
- 3Department of Laboratory Medicine, Ewha Womans University College of Medicine, Seoul, Korea
- 4Department of Diagnostic Immunology, Seegene Medical Foundation, Seoul, Korea
- KMID: 2539195
- DOI: http://doi.org/10.3346/jkms.2023.38.e22
Abstract
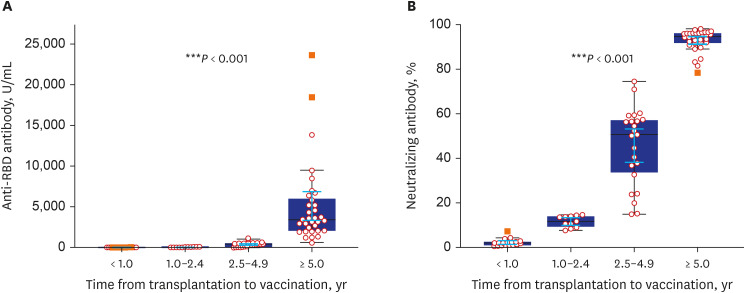
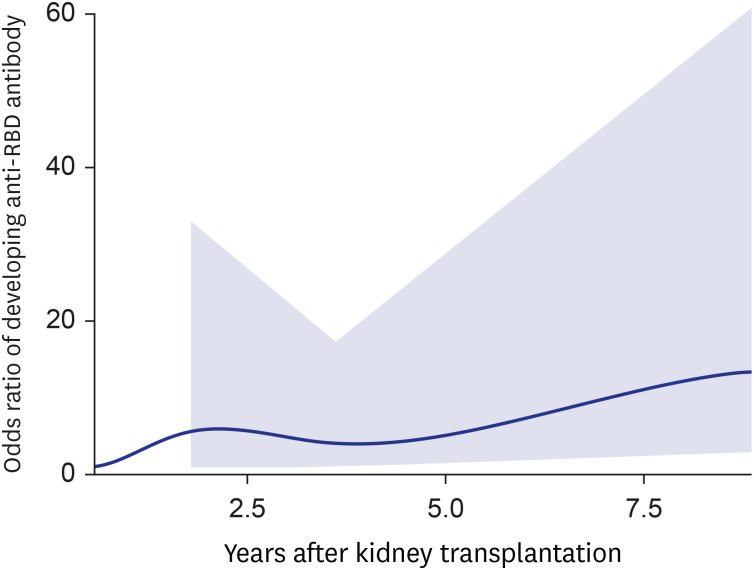
- Eighty-five Korean kidney transplant recipients who received three doses of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine were tested with anti-receptor binding domain (RBD) antibody and neutralizing antibody. High anti-RBD antibody (≥ 100 U/mL) and neutralizing antibody responses (≥ 30%) were detected in 51/85 (60.0%) patients. When we divided the patients with the time from transplantation to vaccination (< 1, 1–2.4, 2.5–4.9, and ≥ 5-year), anti-RBD antibody titers were 3.2 U/mL, 27.8 U/mL, 370.2 U/mL, and 5,094.2 U/mL (P < 0.001) and anti-neutralizing antibody levels were 2.2%, 11.6%, 45.6%, and 93.0% (P < 0.001), respectively. Multivariate analysis revealed increased antibody responses when the time from transplantation to vaccination was five years or longer (odds ratio, 12.0; confidence interval, 2.7–52.8). Korean kidney transplant recipients had suboptimal antibody responses after the third dose of SARS-CoV-2 vaccine. A shorter time from transplantation to vaccination was a risk factor for a low antibody response.
Keyword
Figure
Cited by 1 articles
-
Clinical Utility of Sero-Immunological Responses Against SARS-CoV-2 Nucleocapsid Protein During Subsequent Prevalence of Wild-Type, Delta Variant, and Omicron Variant
Beomki Lee, Jae-Hoon Ko, Jin Yang Baek, Haein Kim, Kyungmin Huh, Sun Young Cho, Cheol-In Kang, Doo Ryeon Chung, Kyong Ran Peck, Eun-Suk Kang
J Korean Med Sci. 2023;38(37):e292. doi: 10.3346/jkms.2023.38.e292.
Reference
-
1. Wei J, Stoesser N, Matthews PC, Ayoubkhani D, Studley R, Bell I, et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021; 6(9):1140–1149. PMID: 34290390.2. Parker EP, Desai S, Marti M, Nohynek H, Kaslow DC, Kochhar S, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022; 10(3):e326–e328. PMID: 35180408.3. Lee AR, Wong SY, Chai LY, Lee SC, Lee MX, Muthiah MD, et al. Efficacy of COVID-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022; 376:e068632. PMID: 35236664.4. Tsapepas D, Paget K, Mohan S, Cohen DJ, Husain SA. Clinically significant COVID-19 following SARS-CoV-2 vaccination in kidney transplant recipients. Am J Kidney Dis. 2021; 78(2):314–317. PMID: 34019949.5. Raja MA, Mendoza MA, Villavicencio A, Anjan S, Reynolds JM, Kittipibul V, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando). 2021; 35(1):100588. PMID: 33246166.6. Nair V, Jandovitz N, Hirsch JS, Abate M, Satapathy SK, Roth N, et al. An early experience on the effect of solid organ transplant status on hospitalized COVID-19 patients. Am J Transplant. 2021; 21(7):2522–2531. PMID: 33443778.7. Sutharattanapong N, Thotsiri S, Kantachuvesiri S, Wiwattanathum P. Benefits of inactivated vaccine and viral vector vaccine immunization on COVID-19 infection in kidney transplant recipients. Vaccines (Basel). 2022; 10(4):572. PMID: 35455322.8. Kang JM, Kim YJ, Huh K, Kim JM, Park WB, Ahn HJ, et al. COVID-19 among solid organ transplant recipients in Korea: surveillance data of the Korean Transplantation Society, January 2020 to March 2022. Korean J Transplant. 2022; 36(2):159–163. PMID: 35919200.9. Parker EPK, Desai S, Marti M, Nohynek H, Kaslow DC, Kochhar S, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022; 10(3):e326–e328. PMID: 35180408.10. Ribas A, Sengupta R, Locke T, Zaidi SK, Campbell KM, Carethers JM, et al. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov. 2021; 11(2):233–236. PMID: 33355178.11. Aydillo T, Gonzalez-Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020; 383(26):2586–2588. PMID: 33259154.12. Efros O, Anteby R, Halfon M, Meisel E, Klang E, Soffer S. Efficacy and safety of third dose of the COVID-19 vaccine among solid organ transplant recipients: a systemic review and meta-analysis. Vaccines (Basel). 2022; 10(1):95. PMID: 35062756.13. Yi SG, Moore LW, Eagar T, Graviss EA, Nguyen DT, Ibrahim H, et al. Risk factors associated with an impaired antibody response in kidney transplant recipients following 2 doses of the SARS-CoV-2 mRNA vaccine. Transplant Direct. 2021; 8(1):e1257. PMID: 34912946.14. Boyarsky BJ, Werbel WA, Avery RK, Tobian AA, Massie AB, Segev DL, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021; 325(21):2204–2206. PMID: 33950155.15. Boyarsky BJ, Werbel WA, Avery RK, Tobian AA, Massie AB, Segev DL, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021; 325(17):1784–1786. PMID: 33720292.16. Hall VG, Ferreira VH, Ku T, Ierullo M, Majchrzak-Kita B, Chaparro C, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021; 385(13):1244–1246. PMID: 34379917.17. Bertrand D, Hamzaoui M, Lemée V, Lamulle J, Laurent C, Etienne I, et al. Antibody and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int. 2021; 100(6):1337–1340. PMID: 34619232.18. Kim N, Minn D, Park S, Roh EY, Yoon JH, Park H, et al. Positivity of SARS-CoV-2 antibodies among Korean healthy healthcare workers 1 and 2 weeks after second dose of Pfizer-BioNTech vaccination. J Korean Med Sci. 2021; 36(21):e158. PMID: 34060264.19. Park JS, Minn D, Hong S, Jeong S, Kim S, Lee CH, et al. Immunogenicity of COVID-19 vaccination in patients with end-stage renal disease undergoing maintenance hemodialysis: the efficacy of a mix-and-match strategy. J Korean Med Sci. 2022; 37(23):e180. PMID: 35698835.20. Carr EJ, Kronbichler A, Graham-Brown M, Abra G, Argyropoulos C, Harper L, et al. Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. Kidney Int Rep. 2021; 6(9):2292–2304. PMID: 34250319.21. Balsby D, Nilsson AC, Möller S, Lindvig SO, Davidsen JR, Abazi R, et al. Determinants of antibody response to a third SARS-CoV-2 mRNA vaccine dose in solid organ transplant Recipients: results from the prospective cohort study COVAC-Tx. Vaccines (Basel). 2022; 10(4):565. PMID: 35455314.22. Masset C, Kerleau C, Garandeau C, Ville S, Cantarovich D, Hourmant M, et al. A third injection of the BNT162b2 mRNA COVID-19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney Int. 2021; 100(5):1132–1135. PMID: 34474075.23. Watanabe M, Balena A, Tuccinardi D, Tozzi R, Risi R, Masi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev. 2022; 38(1):e3465. PMID: 33955644.24. Kang JM, Lee J, Huh KH, Joo DJ, Lee JG, Kim HR, et al. Matched versus mixed COVID-19 vaccinations in korean solid organ transplant recipients: an Observational Study. Transplantation. 2022; 106(9):e392–e403. PMID: 35749755.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity of SARS-CoV-2 vaccine in kidney transplant recipients: a cross-sectional study in Korea
- Seroconversion rates in kidney transplant recipients following SARS-CoV-2 vaccination and its association with immunosuppressive agents: a systematic review and meta-analysis
- Changes in SARS-CoV-2 antibody titers 6 months after the booster dose of BNT162b2 COVID-19 vaccine among health care workers
- Decreased immunogenicity after SARS-CoV-2 vaccination in liver and kidney transplant recipients
- Anti-SARS-CoV-2 spike antibody response to the third dose of BNT162b2 mRNA COVID-19 vaccine and associated factors in Japanese hemodialysis patients