Kosin Med J.
2022 Sep;37(3):176-186. 10.7180/kmj.22.022.
Mucinous carcinoma of the breast: distinctive histopathologic and genetic characteristics
- Affiliations
-
- 1Department of Pathology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- KMID: 2538796
- DOI: http://doi.org/10.7180/kmj.22.022
Abstract
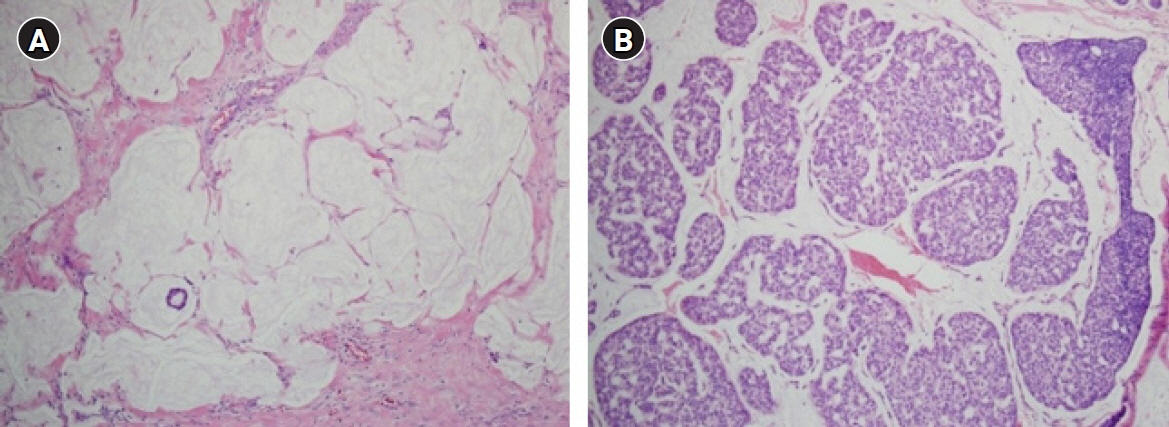
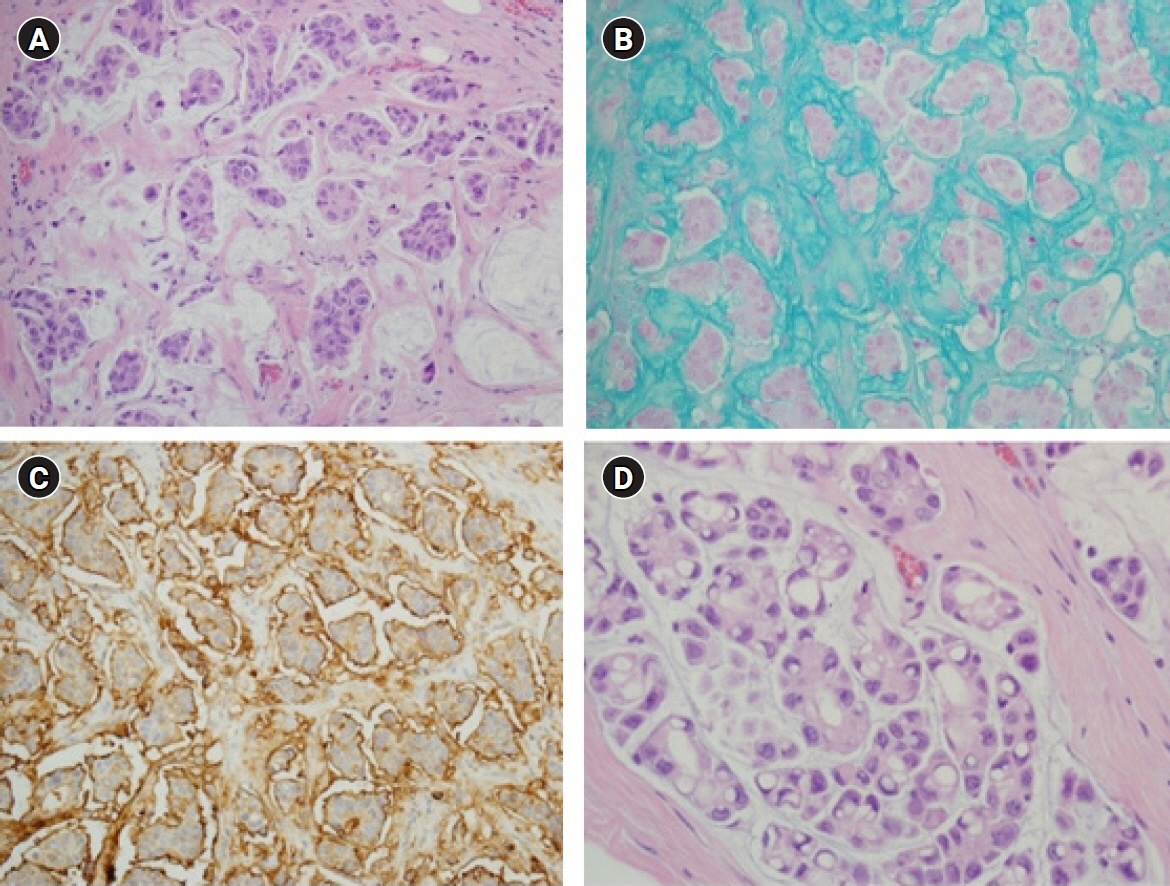
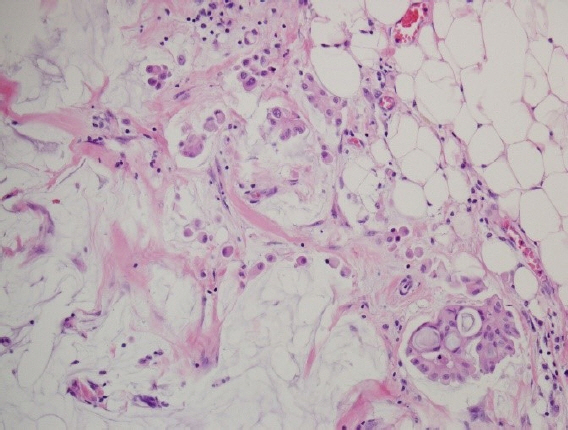
- Mucinous carcinoma is a rare histologic type of breast cancer that, when classified with favorable histology, can be treated with different therapeutic options. This study reviews the histologic findings of mucinous carcinoma that support or exclude favorable histology and emphasizes the necessity of an appropriate gross examination with radiologic findings for an accurate diagnosis. In addition, unusual findings such as micropapillary arrangements and lobular differentiation in mucinous carcinoma and their implications for prognosis and treatment are reviewed. Mucinous carcinoma involves upregulation of MUC2, a mucus-associated gene common in mucinous carcinoma of the breast as well as various other organs. In mucinous carcinoma, the fraction of genome altered and tumor mutation burden are lower than those of invasive carcinoma of no special type, the most common histology of breast cancer. In addition, the genetic alterations found in mucinous carcinoma are diverse, unlike the pathognomonic genetic alterations observed in other histologic types of breast cancer. These genetic features support the importance of conventional microscopic evaluations for the pathologic differential diagnosis of mucinous carcinoma of the breast in routine practice. A variety of breast lesions, including mucinous cystadenocarcinoma and mucocele-like lesions, as well as mucinous carcinoma from other organs, can mimic mucinous carcinoma of the breast. In order to obtain an accurate pathologic diagnosis, careful evaluation of the overall histopathologic characteristics and ancillary testing are required to provide information on appropriate treatment and prognosis.
Keyword
Figure
Reference
-
References
1. Anderson WF, Chu KC, Chang S, Sherman ME. Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2004; 13:1128–35.2. Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008; 111:541–7.3. Kim HS, Lee JU, Yoo TK, Chae BJ, Son D, Kim YJ, et al. Omission of chemotherapy for the treatment of mucinous breast cancer: a nationwide study from the Korean Breast Cancer Society. J Breast Cancer. 2019; 22:599–612.4. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guideline in Oncology (NCCN Guidelines®) Breast Cancer [Internet]. Philadelphia: NCCN;c2022. [cited 2021 Dec 20]. https://www.nccn.org.5. Wen HY, Desmedt C, Reis-Filho JS, Schmitt F. Mucinous carcinoma. In : Allison KH, Brogi E, Ellis IO, Fox S, Morris E, Sahin A, editors. WHO Classification of Tumours, Breast Tumours. 5th ed. Lyon: International Agency for Research on Cancer;2019. p. 123–5.6. Bitencourt AG, Graziano L, Osorio CA, Guatelli CS, Souza JA, Mendonca MH, et al. MRI features of mucinous cancer of the breast: correlation with pathologic findings and other imaging methods. AJR Am J Roentgenol. 2016; 206:238–46.7. Memis A, Ozdemir N, Parildar M, Ustun EE, Erhan Y. Mucinous (colloid) breast cancer: mammographic and US features with histologic correlation. Eur J Radiol. 2000; 35:39–43.8. Monzawa S, Yokokawa M, Sakuma T, Takao S, Hirokaga K, Hanioka K, et al. Mucinous carcinoma of the breast: MRI features of pure and mixed forms with histopathologic correlation. AJR Am J Roentgenol. 2009; 192:W125–31.9. Pintican R, Duma M, Chiorean A, Fetica B, Badan M, Bura V, et al. Mucinous versus medullary breast carcinoma: mammography, ultrasound, and MRI findings. Clin Radiol. 2020; 75:483–96.10. Zhang L, Jia N, Han L, Yang L, Xu W, Chen W. Comparative analysis of imaging and pathology features of mucinous carcinoma of the breast. Clin Breast Cancer. 2015; 15:e147–54.11. Zhou X, Zheng Z, Li Y, Zhao W, Lin Y, Zhang J, et al. The clinical features and prognosis of patients with mucinous breast carcinoma compared with those with infiltrating ductal carcinoma: a population-based study. BMC Cancer. 2021; 21:536.12. Allison KH, Hammond ME, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists guideline update. Arch Pathol Lab Med. 2020; 144:545–63.13. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–95.14. Wolff AC, Hammond ME, Allison KH, Harvey BE, McShane LM, Dowsett M. HER2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update summary. J Oncol Pract. 2018; 14:437–41.15. Tan PH, Tse GM, Bay BH. Mucinous breast lesions: diagnostic challenges. J Clin Pathol. 2008; 61:11–9.16. Capella C, Eusebi V, Mann B, Azzopardi JG. Endocrine differentiation in mucoid carcinoma of the breast. Histopathology. 1980; 4:613–30.17. Tse GM, Ma TK, Chu WC, Lam WW, Poon CS, Chan WC. Neuroendocrine differentiation in pure type mammary mucinous carcinoma is associated with favorable histologic and immunohistochemical parameters. Mod Pathol. 2004; 17:568–72.18. Korean Breast Cancer Society. 2021 the 9th Korean Clinical Practice Guideline for Breast Cancer [Internet]. Seoul: Korean Breast Cancer Society;c2021. [cited 2021 Apr 20]. https://www.kbcs.or.kr/journal/file/211104_2.pdf.19. Barbashina V, Corben AD, Akram M, Vallejo C, Tan LK. Mucinous micropapillary carcinoma of the breast: an aggressive counterpart to conventional pure mucinous tumors. Hum Pathol. 2013; 44:1577–85.20. Ranade A, Batra R, Sandhu G, Chitale RA, Balderacchi J. Clinicopathological evaluation of 100 cases of mucinous carcinoma of breast with emphasis on axillary staging and special reference to a micropapillary pattern. J Clin Pathol. 2010; 63:1043–7.21. Liu F, Yang M, Li Z, Guo X, Lin Y, Lang R, et al. Invasive micropapillary mucinous carcinoma of the breast is associated with poor prognosis. Breast Cancer Res Treat. 2015; 151:443–51.22. Sun P, Zhong Z, Lu Q, Li M, Chao X, Chen D, et al. Mucinous carcinoma with micropapillary features is morphologically, clinically and genetically distinct from pure mucinous carcinoma of breast. Mod Pathol. 2020; 33:1945–60.23. Bal A, Joshi K, Sharma SC, Das A, Verma A, Wig JD. Prognostic significance of micropapillary pattern in pure mucinous carcinoma of the breast. Int J Surg Pathol. 2008; 16:251–6.24. Xu X, Bi R, Shui R, Yu B, Cheng Y, Tu X, et al. Micropapillary pattern in pure mucinous carcinoma of the breast: does it matter or not? Histopathology. 2019; 74:248–55.25. Kim HJ, Park K, Kim JY, Kang G, Gwak G, Park I. Prognostic significance of a micropapillary pattern in pure mucinous carcinoma of the breast: comparative analysis with micropapillary carcinoma. J Pathol Transl Med. 2017; 51:403–9.26. Collins K, Ricci A Jr. Micropapillary variant of mucinous breast carcinoma: a distinct subtype. Breast J. 2018; 24:339–42.27. Lepe M, Kalife ET, Ou J, Quddus MR, Singh K. ‘Inside-out’ p120 immunostaining pattern in invasive micropapillary carcinoma of the breast; additional unequivocal evidence of reversed polarity. Histopathology. 2017; 70:832–4.28. Troxell ML. Reversed MUC1/EMA polarity in both mucinous and micropapillary breast carcinoma. Hum Pathol. 2014; 45:432–4.29. Yu J, Bhargava R, Dabbs DJ. Invasive lobular carcinoma with extracellular mucin production and HER-2 overexpression: a case report and further case studies. Diagn Pathol. 2010; 5:36.30. Cserni G, Floris G, Koufopoulos N, Kovacs A, Nonni A, Regitnig P, et al. Invasive lobular carcinoma with extracellular mucin production: a novel pattern of lobular carcinomas of the breast: clinico-pathological description of eight cases. Virchows Arch. 2017; 471:3–12.31. Singh K, DiazGomez B, Wang Y, Ou J, Hansen K. Invasive lobular carcinoma with extracellular mucin: not all mucinous mammary carcinomas are ductal! Int J Surg Pathol. 2019; 27:55–8.32. Burky MJ, Ray EM, Ollila DW, O’Connor SM, Hertel JD, Calhoun BC. Pleomorphic invasive lobular carcinoma of the breast with extracellular mucin and HER2 amplification. Breast Cancer (Auckl). 2020; 14:1178223420976383.33. Nguyen B, Sanchez-Vega F, Fong CJ, Chatila WK, Boroujeni AM, Pareja F, et al. The genomic landscape of carcinomas with mucinous differentiation. Sci Rep. 2021; 11:9478.34. Desmedt C, Veys I, Nguyen B, Leduc S, Bareche Y, Majjaj S, et al. Molecular characterization of mucinous breast cancers. Cancer Res. 2019; 79(4 Suppl):P3-07-04.35. Nguyen B, Veys I, Leduc S, Bareche Y, Majjaj S, Brown DN, et al. Genomic, transcriptomic, epigenetic, and immune profiling of mucinous breast cancer. J Natl Cancer Inst. 2019; 111:742–6.36. Hugen N, Simons M, Halilovic A, van der Post RS, Bogers AJ, Marijnissen-van Zanten MA, et al. The molecular background of mucinous carcinoma beyond MUC2. J Pathol Clin Res. 2014; 1:3–17.37. Pareja F, Lee JY, Brown DN, Piscuoglio S, Gularte-Merida R, Selenica P, et al. The genomic landscape of mucinous breast cancer. J Natl Cancer Inst. 2019; 111:737–41.38. Thennavan A, Beca F, Xia Y, Recio SG, Allison K, Collins LC, et al. Molecular analysis of TCGA breast cancer histologic types. Cell Genom. 2021; 1:100067.39. Lacroix-Triki M, Suarez PH, MacKay A, Lambros MB, Natrajan R, Savage K, et al. Mucinous carcinoma of the breast is genomically distinct from invasive ductal carcinomas of no special type. J Pathol. 2010; 222:282–98.40. Lu K, Wang X, Zhang W, Ye H, Lao L, Zhou X, et al. Clinicopathological and genomic features of breast mucinous carcinoma. Breast. 2020; 53:130–7.41. Yim HE, Kim JH, Ahn MS, Jung Y, Roh J, Park SH, et al. Clinicopathological and molecular analysis of 45 cases of pure mucinous breast cancer. Front Oncol. 2021; 10:558760.42. Mukherjee A, Russell R, Chin SF, Liu B, Rueda OM, Ali HR, et al. Associations between genomic stratification of breast cancer and centrally reviewed tumour pathology in the METABRIC cohort. NPJ Breast Cancer. 2018; 4:5.43. Wen HY, Desmedt C, Reis-Filho JS, Schmitt F. Mucinous carcinoma. In : Allison KH, Brogi E, Ellis IO, Fox S, Morris E, Sahin A, editors. WHO Classification of Tumours, Breast Tumours. 5th ed. Lyon: International Agency for Research on Cancer;2019. p. 126–7.44. Lin LH, Hernandez O, Zhu K, Guth A, Cotzia P, Darvishian F. Genetic profile of primary mucinous cystadenocarcinoma of the breast: a case report. Breast J. 2021; 27:731–4.45. Kim SE, Park JH, Hong S, Koo JS, Jeong J, Jung WH. Primary mucinous cystadenocarcinoma of the breast: cytologic finding and expression of MUC5 are different from mucinous carcinoma. Korean J Pathol. 2012; 46:611–6.46. Koufopoulos N, Goudeli C, Syrios J, Filopoulos E, Khaldi L. Mucinous cystadenocarcinoma of the breast: the challenge of diagnosing a rare entity. Rare Tumors. 2017; 9:98–100.47. Koenig C, Tavassoli FA. Mucinous cystadenocarcinoma of the breast. Am J Surg Pathol. 1998; 22:698–703.48. Ohi Y, Umekita Y, Rai Y, Sagara Y, Baba S, Tamada S, et al. Mucocele-like lesions of the breast: a long-term follow-up study. Diagn Pathol. 2011; 6:29.49. Kim SJ, Kim JY. Unusual changing calcification patterns on the mammogram in a pure mucocele-like lesion of the breast: a case report. Am J Case Rep. 2019; 20:926–32.50. Park YJ, Kim EK. A pure mucocele-like lesion of the breast diagnosed on ultrasonography-guided core-needle biopsy: is imaging follow-up sufficient? Ultrasonography. 2015; 34:133–8.51. Zhang G, Ataya D, L Lebda P, Calhoun BC. Mucocele-like lesions diagnosed on breast core biopsy: low risk of upgrade and subsequent carcinoma. Breast J. 2018; 24:314–8.52. Meares AL, Frank RD, Degnim AC, Vierkant RA, Frost MH, Hartmann LC, et al. Mucocele-like lesions of the breast: a clinical outcome and histologic analysis of 102 cases. Hum Pathol. 2016; 49:33–8.53. Bombonati A, Lerwill MF. Metastases to and from the breast. Surg Pathol Clin. 2012; 5:719–47.54. Chu PG, Chung L, Weiss LM, Lau SK. Determining the site of origin of mucinous adenocarcinoma: an immunohistochemical study of 175 cases. Am J Surg Pathol. 2011; 35:1830–6.55. Park SY, Kim BH, Kim JH, Lee S, Kang GH. Panels of immunohistochemical markers help determine primary sites of metastatic adenocarcinoma. Arch Pathol Lab Med. 2007; 131:1561–7.56. Dragomir A, de Wit M, Johansson C, Uhlen M, Ponten F. The role of SATB2 as a diagnostic marker for tumors of colorectal origin: results of a pathology-based clinical prospective study. Am J Clin Pathol. 2014; 141:630–8.57. Aldaoud N, Erashdi M, AlKhatib S, Abdo N, Al-Mohtaseb A, Graboski-Bauer A. The utility of PAX8 and SATB2 immunohistochemical stains in distinguishing ovarian mucinous neoplasms from colonic and appendiceal mucinous neoplasm. BMC Res Notes. 2019; 12:770.58. Wendroth SM, Mentrikoski MJ, Wick MR. GATA3 expression in morphologic subtypes of breast carcinoma: a comparison with gross cystic disease fluid protein 15 and mammaglobin. Ann Diagn Pathol. 2015; 19:6–9.59. El Hag MI, Hag AM, Ha JP, Michael CW. Comparison of GATA-3, mammaglobin, GCDFP-15 expression in breast carcinoma in serous effusions: a cell-block micro-array study. Pleura Peritoneum. 2017; 2:143–8.60. Hu A, Li H, Zhang L, Ren C, Wang Y, Liu Y, et al. Differentiating primary and extragenital metastatic mucinous ovarian tumours: an algorithm combining PAX8 with tumour size and laterality. J Clin Pathol. 2015; 68:522–8.61. Strickland S, Wasserman JK, Giassi A, Djordjevic B, Parra-Herran C. Immunohistochemistry in the diagnosis of mucinous neoplasms involving the ovary: the added value of SATB2 and biomarker discovery through protein expression database mining. Int J Gynecol Pathol. 2016; 35:191–208.62. Sun F, Wang P, Zheng Y, Jia W, Liu F, Xiao W, et al. Diagnosis, clinicopathological characteristics and prognosis of pulmonary mucinous adenocarcinoma. Oncol Lett. 2018; 15:489–94.63. Wu J, Chu PG, Jiang Z, Lau SK. Napsin A expression in primary mucin-producing adenocarcinomas of the lung: an immunohistochemical study. Am J Clin Pathol. 2013; 139:160–6.64. Kim MJ. The usefulness of CDX-2 for differentiating primary and metastatic ovarian carcinoma: an immunohistochemical study using a tissue microarray. J Korean Med Sci. 2005; 20:643–8.65. Ma L, Wolkow N, Jakobiec FA. Choroidal mucinous metastatic adenocarcinoma from the colon: a diagnostic challenge. Ocul Oncol Pathol. 2019; 5:66–74.66. Liu F, Gao Z, Shen D, Zhao H, Wang C, Ye Y, et al. Significance of SATB2 expression in colon cancer and its differential diagnosis in digestive tract adenocarcinoma and ovarian primary and metastatic carcinoma. Pathol Res Pract. 2019; 215:152430.67. Nonaka D, Chiriboga L, Soslow RA. Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. 2008; 32:1566–71.68. Pinto PB, Derchain SF, Andrade LA. Metastatic mucinous carcinomas in the ovary: a practical approach to diagnosis related to gross aspects and to immunohistochemical evaluation. Int J Gynecol Pathol. 2012; 31:313–8.69. Vang R, Gown AM, Barry TS, Wheeler DT, Yemelyanova A, Seidman JD, et al. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am J Surg Pathol. 2006; 30:1130–9.70. Vang R, Gown AM, Wu LS, Barry TS, Wheeler DT, Yemelyanova A, et al. Immunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: comparison with CK20 and correlation with coordinate expression of CK7. Mod Pathol. 2006; 19:1421–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fine Needle Aspiration Cytology of Solid Papillary Carcinoma of the Breast: Report of a case associated with mucinous carcinoma
- Mucinous Carcinoma of the Breast
- Mucinous precursor lesions of mucinous carcinoma in breast: Incidence and histopathologic features
- The Expression of Glut-1, CAIX, and MCT4 in Mucinous Carcinoma
- MR Imaging of Mucinous Carcinoma of the Breast Associated with Ductal Carcinoma In Situ: Case Report