Cancer Res Treat.
2023 Jan;55(1):73-82. 10.4143/crt.2021.1202.
Analysis of Once-Daily Thoracic Radiotherapy Dose According to the Underlying Lung Disease in Patients with Limited-Stage Small Cell Lung Cancer Undergoing Concurrent Chemoradiotherapy
- Affiliations
-
- 1Department of Radiation Oncology, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea
- 2Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Radiation Oncology, Seoul National University Hospital, Seoul, Korea
- 4Institute of Radiation Medicine, Medical Research Center, Seoul National University, Seoul, Korea
- 5Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2537993
- DOI: http://doi.org/10.4143/crt.2021.1202
Abstract
- Purpose
In the treatment of concurrent chemoradiotherapy (CCRT) in limited-stage small cell lung cancer, the optimal once-daily radiotherapy (RT) dose/fractionation remain unclear although it is the most frequently used. Therefore, this study aimed to compare the treatment outcomes and toxicities of modest dose RT (≤ 54 Gy) with those of standard dose RT (> 54 Gy) and investigate the benefit of the high dose based on patient factors.
Materials and Methods
Since 2004, our institution has gradually increased the thoracic RT dose. Among the 225 patients who underwent CCRT, 84 patients (37.3%) received > 54 Gy. Because the patients treated with RT > 54 Gy were not randomly assigned, propensity score matching (PSM) was performed.
Results
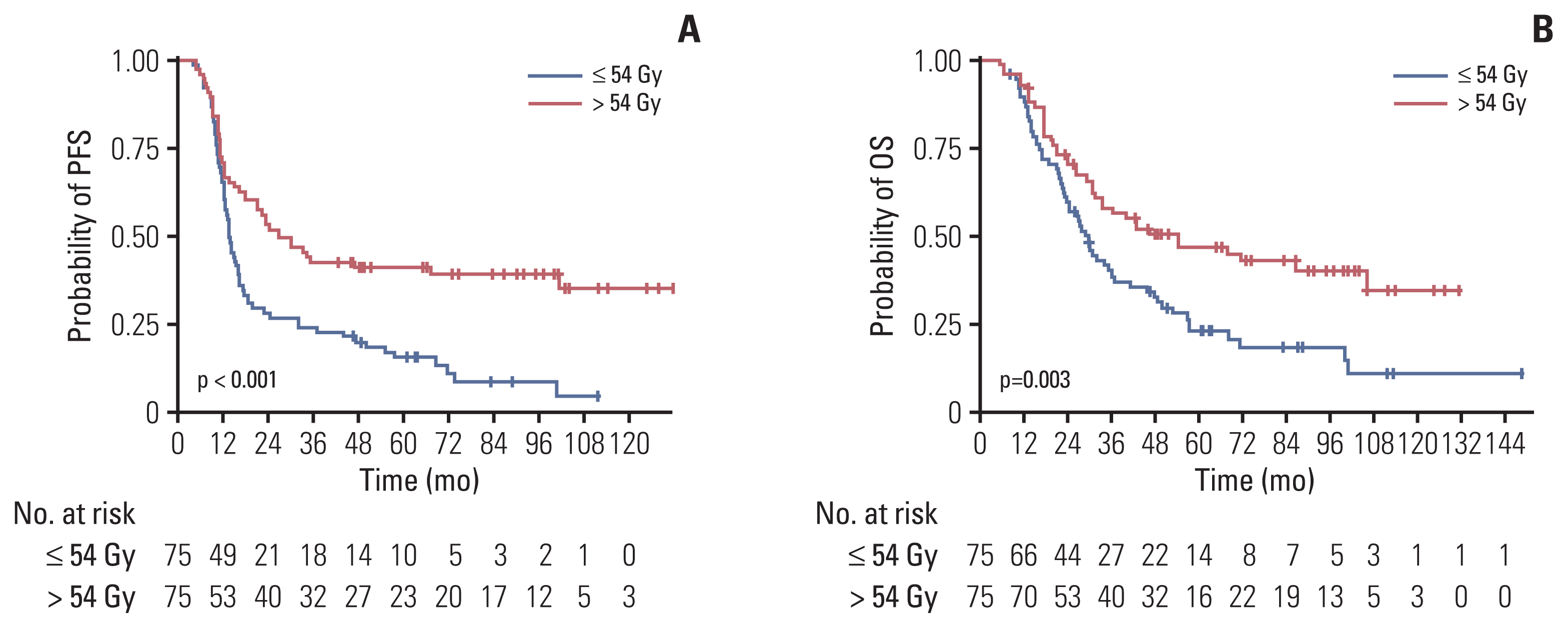
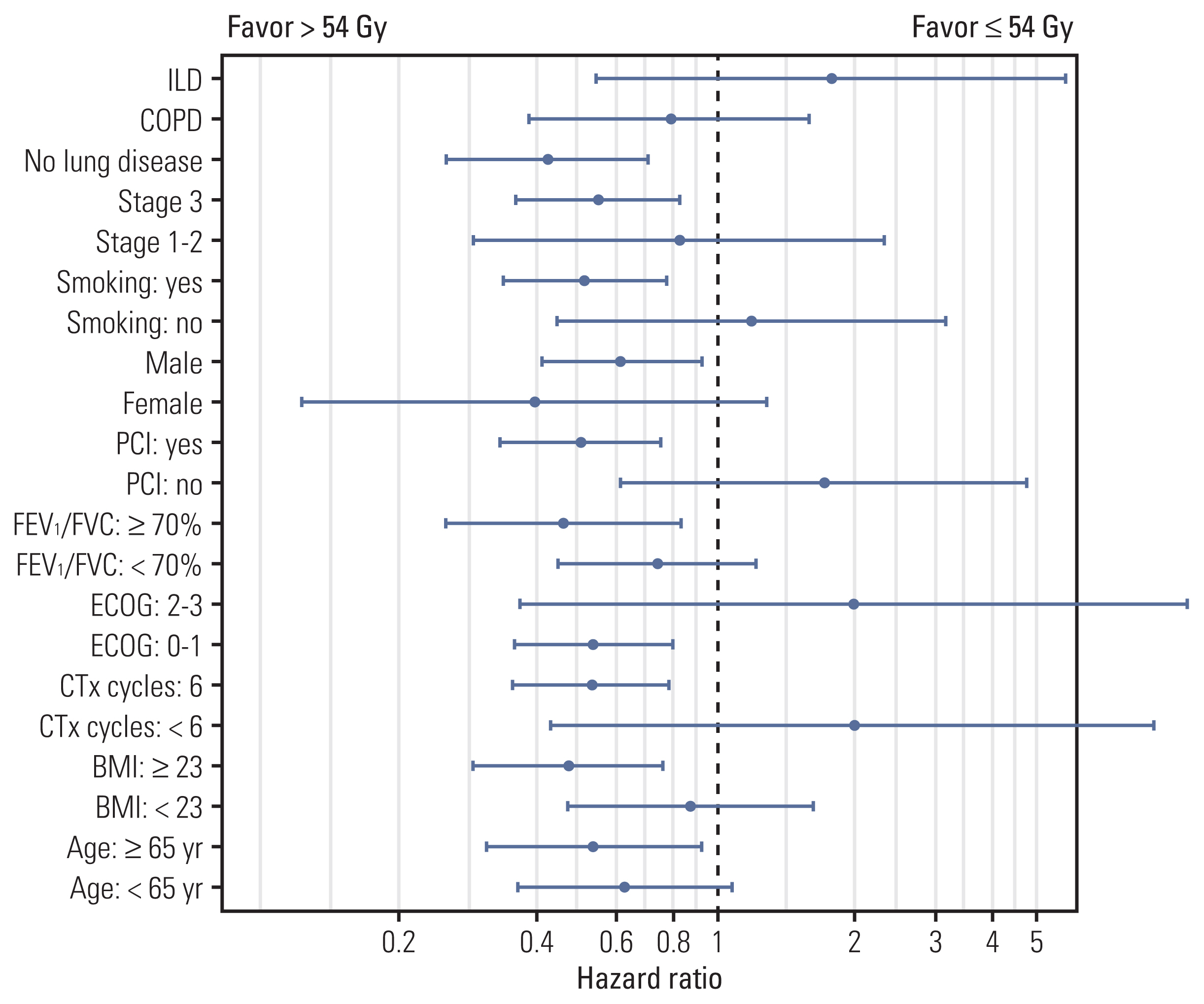
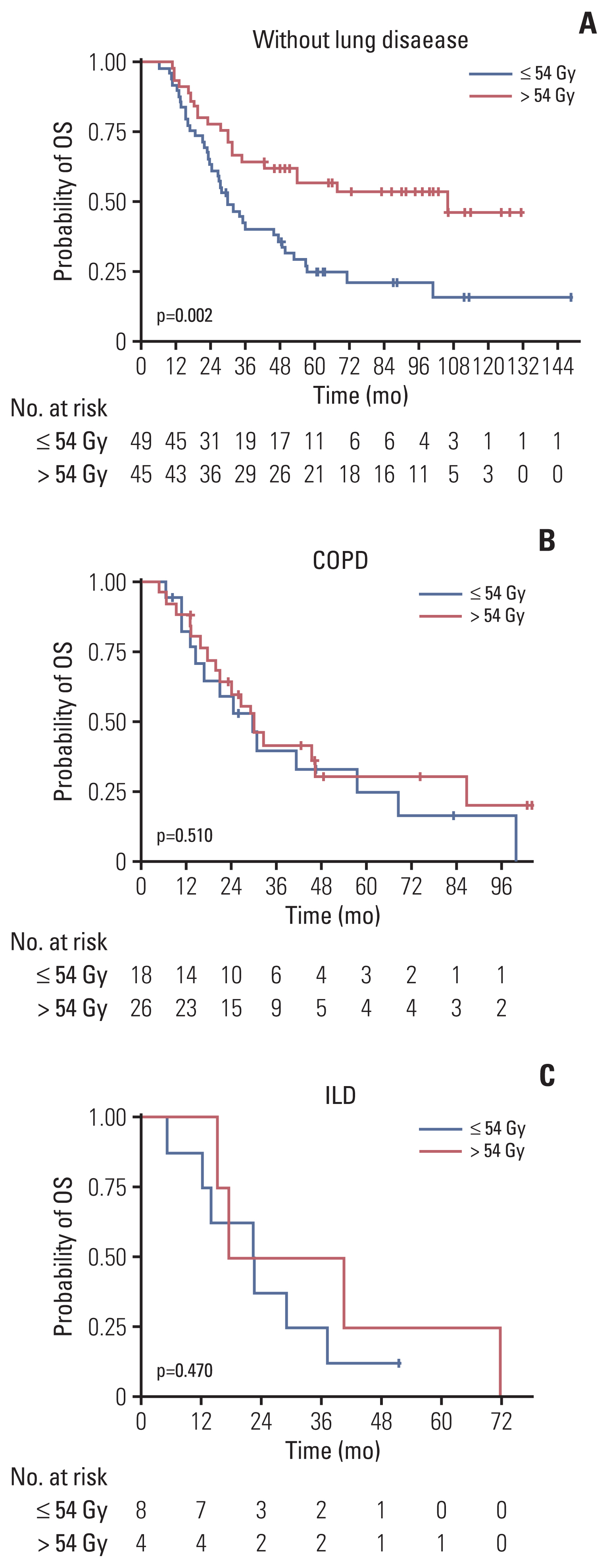
The proportion of patients treated with > 54 Gy increased over time (p=0.014). Multivariate analysis revealed that the overall tumor stage and dose > 54 Gy (hazard ratio, 0.65; p=0.029) were independent prognostic factors for overall survival (OS). PSM confirmed that thoracic RT doses of > 54 Gy showed significantly improved progression-free survival (3-year, 42.7% vs. 24.0%; p < 0.001) and OS (3-year, 56.2% vs. 38.5%; p=0.003). Sensitivity analysis also showed that 60 Gy resulted in better survival than 54 Gy. However, in patients with underlying lung disease, OS benefit from > 54 Gy was not observed but considerable rates of severe pulmonary toxicities were observed (p=0.001).
Conclusion
Our analysis supports that the 60 Gy RT dose should be considered in the once-daily regimen of CCRT for limited-stage small cell lung cancer without underlying lung disease, but RT dose > 54 Gy did not seem to benefit for patients with chronic obstructive pulmonary disease or interstitial lung disease. Further study is needed to validate these results.
Figure
Reference
-
References
1. Turrisi AT 3rd, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999; 340:265–71.2. Schreiber D, Wong AT, Schwartz D, Rineer J. Utilization of hyperfractionated radiation in small-cell lung cancer and its impact on survival. J Thorac Oncol. 2015; 10:1770–5.3. Farrell MJ, Yahya JB, Degnin C, Chen Y, Holland JM, Henderson MA, et al. Radiation dose and fractionation for limited-stage small-cell lung cancer: survey of US radiation oncologists on practice patterns. Clin Lung Cancer. 2019; 20:13–9.4. Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017; 18:1116–25.5. Bogart JA, Wang XF, Masters GA, Gao J, Komaki R, Kuzma CS, et al. Phase 3 comparison of high-dose once-daily (QD) thoracic radiotherapy (TRT) with standard twice-daily (BID) TRT in limited stage small cell lung cancer (LSCLC): CALGB 30610 (Alliance)/RTOG 0538. J Clin Oncol. 2021; 39(15 Suppl):8505.6. Daly ME, Ismaila N, Decker RH, Higgins K, Owen D, Saxena A, et al. Radiation therapy for small-cell lung cancer: ASCO guideline endorsement of an ASTRO guideline. J Clin Oncol. 2021; 39:931–9.7. Stinchcombe TE, Fan W, Schild SE, Vokes EE, Bogart J, Le QT, et al. A pooled analysis of individual patient data from National Clinical Trials Network clinical trials of concurrent chemoradiotherapy for limited-stage small cell lung cancer in elderly patients versus younger patients. Cancer. 2019; 125:382–90.8. Kobayashi H, Wakuda K, Naito T, Mamesaya N, Omori S, Ono A, et al. Chemoradiotherapy for limited-stage small-cell lung cancer and interstitial lung abnormalities. Radiat Oncol. 2021; 16:52.9. Han TJ, Kim HJ, Wu HG, Heo DS, Kim YW, Lee SH. Comparison of treatment outcomes between involved-field and elective nodal irradiation in limited-stage small cell lung cancer. Jpn J Clin Oncol. 2012; 42:948–54.10. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995; 31:1341–6.11. Li F, Zhou Z, Wu A, Cai Y, Wu H, Chen M, et al. Preexisting radiological interstitial lung abnormalities are a risk factor for severe radiation pneumonitis in patients with small-cell lung cancer after thoracic radiation therapy. Radiat Oncol. 2018; 13:82.12. Song MJ, Lim SY, Park JS, Yoon HI, Lee JH, Kim SY, et al. Prognosis of small cell lung cancer with idiopathic pulmonary fibrosis: assessment according to GAP stage. J Oncol. 2019; 2019:5437390.13. Kim K, Moon S, Kim Y, Kim T, Cho K, Han J, et al. Treatment outcomes of limited-stage small cell lung cancer patients treated with concurrent chemoradiation therapy: a comparative analysis of different radiation dose fractionation schedules in a single institution. Int J Radiat Oncol. 2014; 90(1 Suppl):S632.14. Han D, Hao S, Tao C, Zhao Q, Wei Y, Song Z, et al. Comparison of once daily radiotherapy to 60 Gy and twice daily radiotherapy to 45 Gy for limited stage small-cell lung cancer. Thorac Cancer. 2015; 6:643–8.15. Watkins JM, Fortney JA, Wahlquist AE, Shirai K, Garrett-Mayer E, Aguero EG, et al. Once-daily radiotherapy to > or =59.4 Gy versus twice-daily radiotherapy to > or =45.0 Gy with concurrent chemotherapy for limited-stage small-cell lung cancer: a comparative analysis of toxicities and outcomes. Jpn J Radiol. 2010; 28:340–8.16. Gazula A, Baldini EH, Chen A, Kozono D. Comparison of once and twice daily radiotherapy for limited stage small-cell lung cancer. Lung. 2014; 192:151–8.17. Tomita N, Kodaira T, Hida T, Tachibana H, Nakamura T, Nakahara R, et al. The impact of radiation dose and fractionation on outcomes for limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2010; 76:1121–6.18. Bogart J, Wang X, Masters G, Zhu H, Komaki R, Gaspar L, et al. Interim toxicity analysis for patients with limited stage small cell lung cancer (LSCLC) treated on the experimental thoracic radiotherapy (TRT) arms of CALGB 30610 (Alliance)/RTOG 0538. Ann Oncol. 2019; 30:v711.19. Bogart JA, Wang X, Masters GA, Gao J, Komaki R, Gaspar LE, et al. Short communication: interim toxicity analysis for patients with limited stage small cell lung cancer (LSCLC) treated on CALGB 30610 (Alliance)/RTOG 0538. Lung Cancer. 2021; 156:68–71.20. Gronberg BH, Killingberg KT, Flotten O, Brustugun OT, Hornslien K, Madebo T, et al. High-dose versus standard-dose twice-daily thoracic radiotherapy for patients with limited stage small-cell lung cancer: an open-label, randomised, phase 2 trial. Lancet Oncol. 2021; 22:321–31.21. Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE, et al. Long-term results of NRG oncology RTOG 0617: standard-versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2020; 38:706–14.22. Komaki R, Swann RS, Ettinger DS, Glisson BS, Sandler AB, Movsas B, et al. Phase I study of thoracic radiation dose escalation with concurrent chemotherapy for patients with limited small-cell lung cancer: report of Radiation Therapy Oncology Group (RTOG) protocol 97–12. Int J Radiat Oncol Biol Phys. 2005; 62:342–50.23. Rutter CE, Park HS, Corso CD, Yeboa DN, Mancini BR, Lester-Coll NH, et al. Comparison of survival outcomes among standard radiotherapy regimens in limited-stage small cell lung cancer patients receiving concurrent chemoradiation. Lung Cancer. 2015; 90:243–8.24. Zayed S, Chen H, Ali E, Rodrigues GB, Warner A, Palma DA, et al. Is there a role for hypofractionated thoracic radiation therapy in limited-stage small cell lung cancer? A propensity score matched analysis. Int J Radiat Oncol Biol Phys. 2020; 108:575–86.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Definitive Radiotherapy of Non-Small Cell Lung Cancer
- Comparison between <60 Gy and > or =60 Gy Once-Daily Thoracic Irradiation for Patients with Limited-stage Smallcell Lung Cancer
- Impact of radiation dose on concurrent chemoradiotherapy for limited-stage small-cell lung cancer
- Factors predicting radiation pneumonitis in locally advanced non-small cell lung cancer
- Chemotherapy for Small Cell Lung Cancer