Korean J Physiol Pharmacol.
2023 Jan;27(1):31-38. 10.4196/kjpp.2023.27.1.31.
Characterization of a conjugated polysuccinimide-carboplatin compound
- Affiliations
-
- 1Department of Radiation Oncology, Jeonbuk National University Medical School, Jeonju 54907, Korea
- 2Research Institute of Clinical Medicine of Jeonbuk National University-Biomedical Research Institute of Jeonbuk National University Hospital, Jeonju 54907, Korea
- 3CELLDI Co., Ltd, Cheongju 28160, Korea
- 4Laboratory of Nanochemistry, Department of Biophysics and Radiation Biology, Semmelweis University, Budapest 1089, Hungary
- 5Department of Biochemistry & Cell Biology, School of Medicine, Kyungpook National University, Daegu 41940, Korea
- 6Department of Obstetrics and Gynecology, Jeonbuk National University Medical School, Jeonju 54907, Korea
- KMID: 2537501
- DOI: http://doi.org/10.4196/kjpp.2023.27.1.31
Abstract
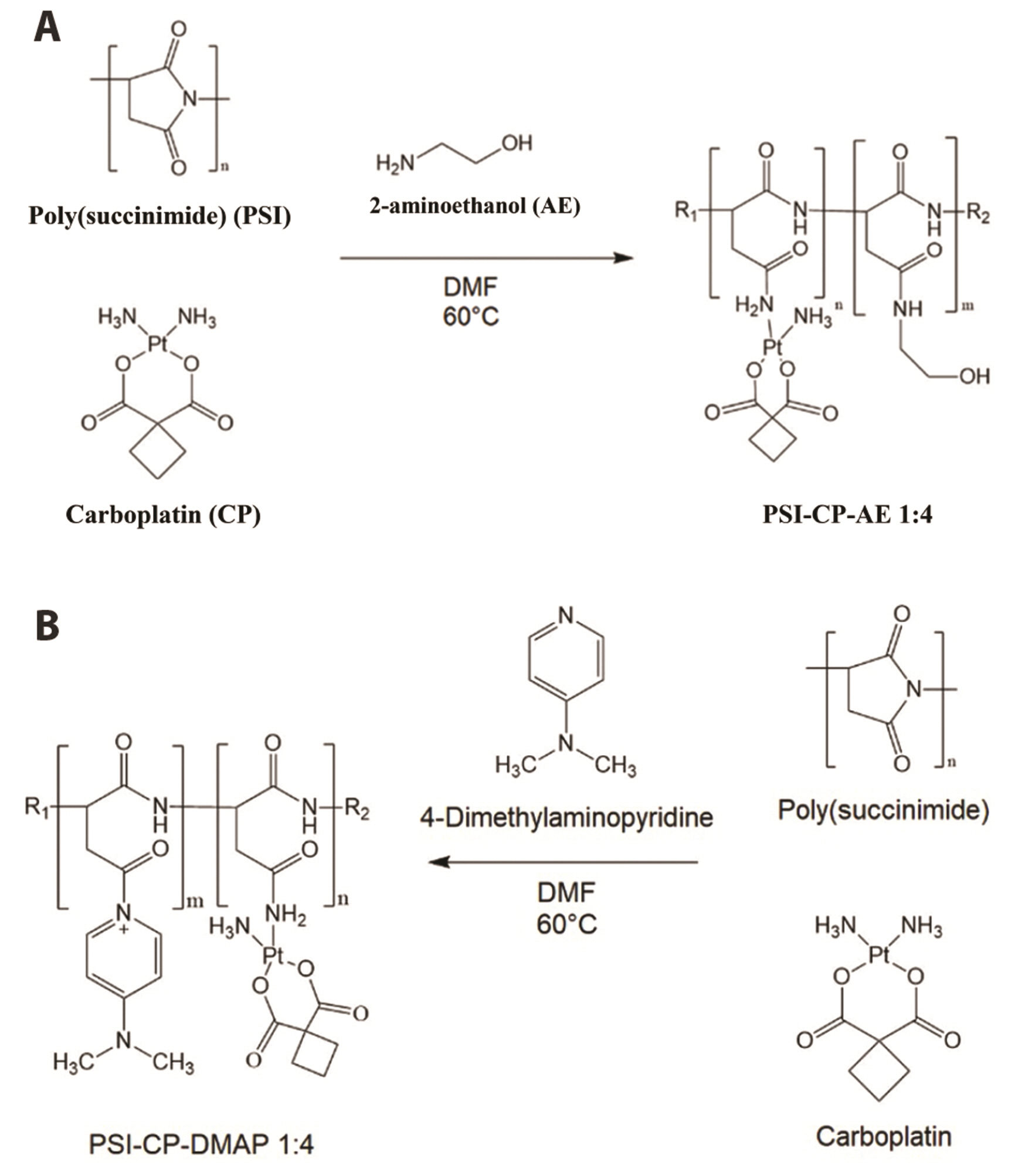
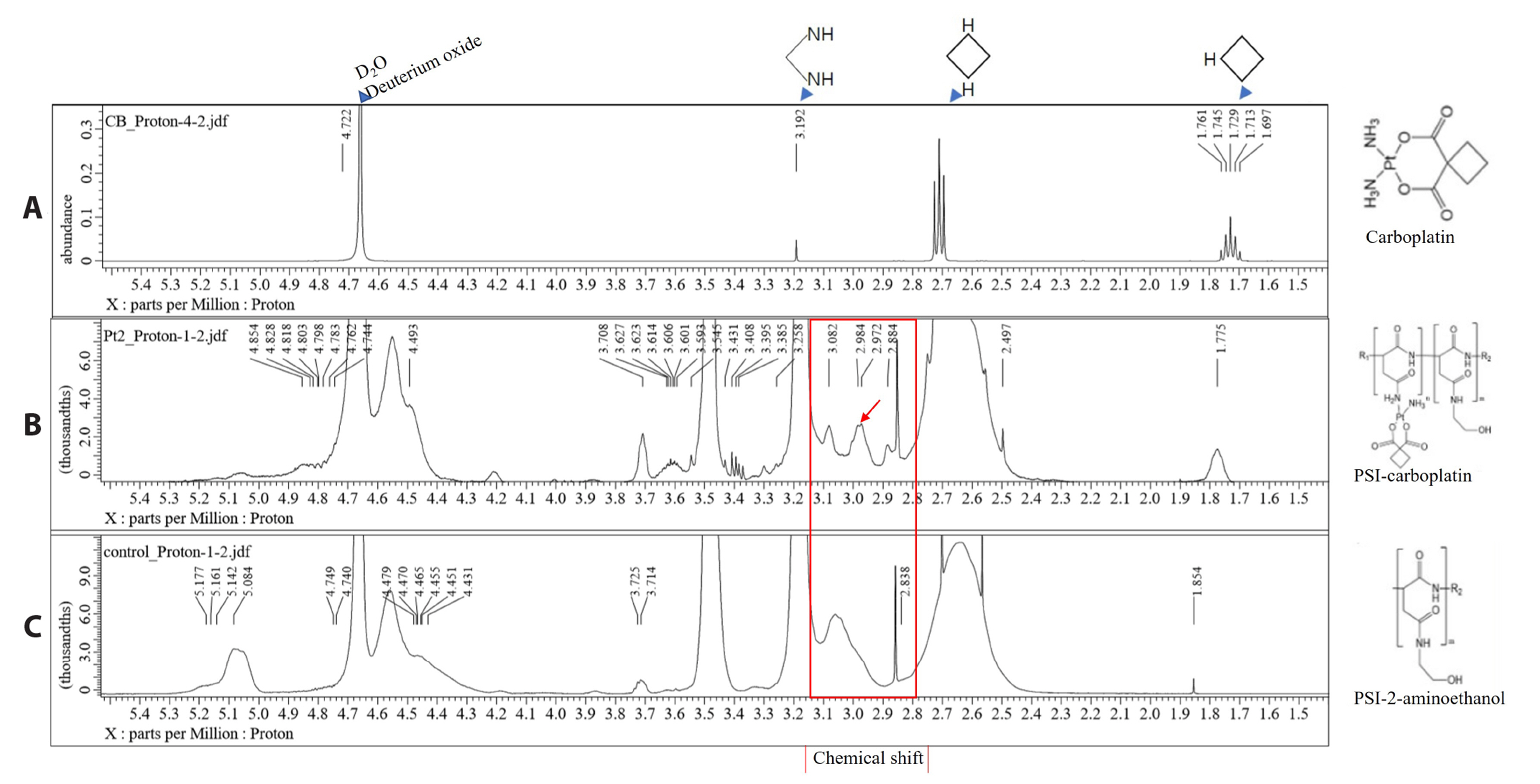
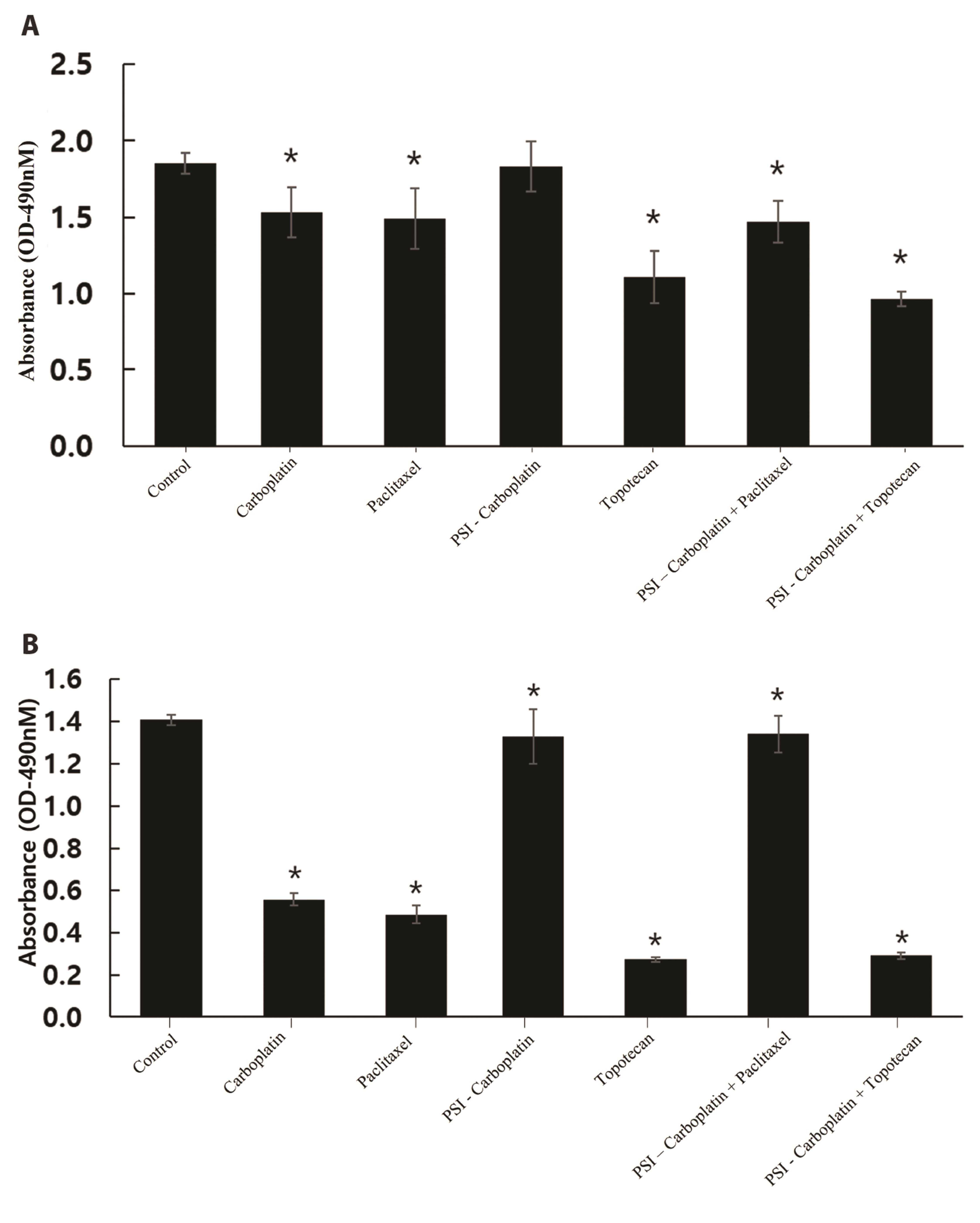
- Carboplatin, an advanced anticancer drug with excellent efficacy against ovarian cancer, was developed to alleviate the side effects that often occur with cisplatin and other platinum-based compounds. Our study reports the in vitro characteristics, viability, and activity of cells expressing the inducible nitric oxide synthase (iNOS) gene after carboplatin was conjugated with polysuccinimide (PSI) and administered in combination with other widely used anticancer drugs. PSI, which has promising properties as a drug delivery material, could provide a platform for prolonging carboplatin release, regulating its dosage, and improving its side effects. The iNOS gene has been shown to play an important role in both cancer cell survival and inhibition. Herein, we synthesized a PSI-carboplatin conjugate to create a modified anticancer agent and confirmed its successful conjugation. To ensure its solubility in water, we further modified the structure of the PSI-carboplatin conjugate with 2-aminoethanol groups. To validate its biological characteristics, the ovarian cancer cell line SKOV-3 and normal ovarian Chinese hamster ovary cells were treated with the PSI-carboplatin conjugate alone and in combination with paclitaxel and topotecan, both of which are used in conventional chemotherapy. Notably, PSI-carboplatin conjugation can be used to predict changes in the genes involved in cancer growth and inhibition. In conclusion, combination treatment with the newly synthesized polymer-carboplatin conjugate and paclitaxel displayed anticancer activity against ovarian cancer cells but was not toxic to normal ovarian cancer cells, resulting in the development of an effective candidate anticancer drug without severe side effects.
Keyword
Figure
Reference
-
1. Reid BM, Permuth JB, Sellers TA. 2017; Epidemiology of ovarian cancer: a review. Cancer Biol Med. 14:9–32. DOI: 10.20892/j.issn.2095-3941.2016.0084. PMID: 28443200. PMCID: PMC5365187. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85017143290&origin=inward.2. Kujawa KA, Lisowska KM. 2015; Ovarian cancer--from biology to clinic. Postepy Hig Med Dosw (Online). 69:1275–1290. Polish. DOI: 10.5604/17322693.1184451. PMID: 26671919. PMCID: PMC5365187. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84973411296&origin=inward.3. Webb PM, Jordan SJ. 2017; Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 41:3–14. DOI: 10.1016/j.bpobgyn.2016.08.006. PMID: 27743768. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85008698217&origin=inward.4. Karam A, Ledermann JA, Kim JW, Sehouli J, Lu K, Gourley C, Katsumata N, Burger RA, Nam BH, Bacon M, Ng C, Pfisterer J, Bekkers RLM, Casado Herráez A, Redondo A, Fujiwara H, Gleeson N, Rosengarten O, Scambia G, Zhu J, et al. 2017; Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: first-line interventions. Ann Oncol. 28:711–717. DOI: 10.1093/annonc/mdx011. PMID: 28327917. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85021858496&origin=inward.5. Potara M, Nagy-Simon T, Craciun AM, Suarasan S, Licarete E, Imre-Lucaci F, Astilean S. 2017; Carboplatin-loaded, Raman-encoded, chitosan-coated silver nanotriangles as multimodal traceable nanotherapeutic delivery systems and pH reporters inside human ovarian cancer cells. ACS Appl Mater Interfaces. 9:32565–32576. DOI: 10.1021/acsami.7b10075. PMID: 28872817. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85030162417&origin=inward.6. Wang Y, Wang L, Chen G, Gong S. 2017; Carboplatin-complexed and cRGD-conjugated unimolecular nanoparticles for targeted ovarian cancer therapy. Macromol Biosci. 17:1600292. DOI: 10.1002/mabi.201600292. PMID: 27911475. PMCID: PMC5914160. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85006307295&origin=inward.7. Coleman RL. 2002; Emerging role of topotecan in front-line treatment of carcinoma of the ovary. Oncologist. 7 Suppl 5:46–55. DOI: 10.1634/theoncologist.7-suppl_5-46. PMID: 12324633. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036388369&origin=inward.8. Zhang H, Jia L, Xu Y, Zhou XC, Kong B, Li D. 2012; Topotecan plus carboplatin and paclitaxel in first-line treatment of advanced ovarian cancer: a meta-analysis of randomized controlled trials. J Chemother. 24:67–73. DOI: 10.1179/1120009X12Z.0000000002. PMID: 22546760. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84866256593&origin=inward.9. Skarlos DV, Aravantinos G, Kosmidis P, Athanassiadis A, Stathopoulos GP, Pavlidis N, Bafaloukos D, Karphathios S, Papakostas P, Bamia C, Fountzilas G. 1997; Paclitaxel with carboplatin versus paclitaxel with carboplatin alternating with cisplatin as first-line chemotherapy in advanced epithelial ovarian cancer: preliminary results of a Hellenic Cooperative Oncology Group study. Semin Oncol. 24(5 Suppl 15):S15-57–S15-61. PMID: 9346224. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=9844262807&origin=inward.10. Han ES, Wen W, Dellinger TH, Wu J, Lu SA, Jove R, Yim JH. 2018; Ruxolitinib synergistically enhances the anti-tumor activity of paclitaxel in human ovarian cancer. Oncotarget. 9:24304–24319. DOI: 10.18632/oncotarget.24368. PMID: 29849942. PMCID: PMC5966246.11. Ho GY, Woodward N, Coward JI. 2016; Cisplatin versus carboplatin: comparative review of therapeutic management in solid malignancies. Crit Rev Oncol Hematol. 102:37–46. DOI: 10.1016/j.critrevonc.2016.03.014. PMID: 27105947. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84963800478&origin=inward.12. Ledermann JA. 2017; Front-line therapy of advanced ovarian cancer: new approaches. Ann Oncol. 28(suppl_8):viii46–viii50. DOI: 10.1093/annonc/mdx452. PMID: 29232475. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85039553675&origin=inward.13. Shenoi RA, Lai BF, Imran ul-haq M, Brooks DE, Kizhakkedathu JN. 2013; Biodegradable polyglycerols with randomly distributed ketal groups as multi-functional drug delivery systems. Biomaterials. 34:6068–6081. DOI: 10.1016/j.biomaterials.2013.04.043. PMID: 23688604. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84878532612&origin=inward.14. Joye IJ, McClements DJ. 2016; Biopolymer-based delivery systems: challenges and opportunities. Curr Top Med Chem. 16:1026–1039. DOI: 10.2174/1568026615666150825143130. PMID: 26303423. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84959573045&origin=inward.15. Tibbitt MW, Dahlman JE, Langer R. 2016; Emerging frontiers in drug delivery. J Am Chem Soc. 138:704–717. DOI: 10.1021/jacs.5b09974. PMID: 26741786. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84956787698&origin=inward.16. Varga Z, Molnár K, Torma V, Zrínyi M. 2010; Kinetics of volume change of poly(succinimide) gels during hydrolysis and swelling. Phys Chem Chem Phys. 12:12670–12675. DOI: 10.1039/c0cp00527d. PMID: 20730186. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77957923369&origin=inward.17. Sadeghi M, Hemmati S, Hamishehkar H. 2016; Synthesis of a novel superdisintegrant by starch derivatization with polysuccinimide and its application for the development of Ondansetron fast dissolving tablet. Drug Dev Ind Pharm. 42:769–775. DOI: 10.3109/03639045.2015.1075029. PMID: 26289005. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84968546844&origin=inward.18. Juriga D, Laszlo I, Ludanyi K, Klebovich I, Chae CH, Zrinyi M. 2018; Kinetics of dopamine release from poly(aspartamide)-based prodrugs. Acta Biomater. 76:225–238. DOI: 10.1016/j.actbio.2018.06.030. PMID: 29940369. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85049303521&origin=inward.19. Yendluri R, Lvov Y, de Villiers MM, Vinokurov V, Naumenko E, Tarasova E, Fakhrullin R. 2017; Paclitaxel encapsulated in halloysite clay nanotubes for intestinal and intracellular delivery. J Pharm Sci. 106:3131–3139. DOI: 10.1016/j.xphs.2017.05.034. PMID: 28600185. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85026483844&origin=inward.20. Doppalapudi S, Jain A, Domb AJ, Khan W. 2016; Biodegradable polymers for targeted delivery of anti-cancer drugs. Expert Opin Drug Deliv. 13:891–909. DOI: 10.1517/17425247.2016.1156671. PMID: 26983898. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84961210141&origin=inward.21. Nicolas J, Couvreur P. 2017; Polymer nanoparticles for the delivery of anticancer drug. Med Sci (Paris). 33:11–17. French. DOI: 10.1051/medsci/20173301003. PMID: 28120750. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85010748894&origin=inward.22. Velazco-de-la-Garza J, Avérous L, Sosa-Santillán GdJ, Pollet E, Zugasti-Cruz A, Sierra-Rivera CA, Pérez-Aguilar NV, Oyervides-Muñoz E. 2020; Biological properties of novel polysuccinimide derivatives synthesized via quaternary ammonium grafting. Eur Polym J. 131:109705. https://doi.org/10.1016/j.eurpolymj.2020.109705. DOI: 10.1016/j.eurpolymj.2020.109705. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85083468448&origin=inward.23. Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S. 1995; Roles of nitric oxide in tumor growth. Proc Natl Acad Sci U S A. 92:4392–4396. DOI: 10.1073/pnas.92.10.4392. PMID: 7538668. PMCID: PMC41950. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029079394&origin=inward.24. Xie K, Huang S. 2003; Contribution of nitric oxide-mediated apoptosis to cancer metastasis inefficiency. Free Radic Biol Med. 34:969–986. DOI: 10.1016/S0891-5849(02)01364-3. PMID: 12684082. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037446221&origin=inward.25. Brüne B. 2003; Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 10:864–869. DOI: 10.1038/sj.cdd.4401261. PMID: 12867993. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0042665423&origin=inward.26. Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, Felley-Bosco E, Wang XW, Geller DA, Tzeng E, Billiar TR, Harris CC. 1996; Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc Natl Acad Sci U S A. 93:2442–2447. DOI: 10.1073/pnas.93.6.2442. PMID: 8637893. PMCID: PMC39816. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=9244232804&origin=inward.27. Rao CV. 2004; Nitric oxide signaling in colon cancer chemoprevention. Mutat Res. 555:107–119. DOI: 10.1016/j.mrfmmm.2004.05.022. PMID: 15476855. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=5144230475&origin=inward.28. Chazotte-Aubert L, Hainaut P, Ohshima H. 2000; Nitric oxide nitrates tyrosine residues of tumor-suppressor p53 protein in MCF-7 cells. Biochem Biophys Res Commun. 267:609–613. DOI: 10.1006/bbrc.1999.2003. PMID: 10631110. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034685015&origin=inward.29. Li J, Billiar TR, Talanian RV, Kim YM. 1997; Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem Biophys Res Commun. 240:419–424. DOI: 10.1006/bbrc.1997.7672. PMID: 9388494. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031576527&origin=inward.30. Messmer UK, Brüne B. 1996; Nitric oxide-induced apoptosis: p53-dependent and p53-independent signalling pathways. Biochem J. 319(Pt 1):299–305. DOI: 10.1042/bj3190299. PMID: 8870682. PMCID: PMC1217768. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029812216&origin=inward.31. Kielbik M, Szulc-Kielbik I, Klink M. 2019; The potential role of iNOS in ovarian cancer progression and chemoresistance. Int J Mol Sci. 20:1751. DOI: 10.3390/ijms20071751. PMID: 30970628. PMCID: PMC6479373. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064722293&origin=inward.32. Morand S, Devanaboyina M, Staats H, Stanbery L, Nemunaitis J. 2021; Ovarian cancer immunotherapy and personalized medicine. Int J Mol Sci. 22:6532. DOI: 10.3390/ijms22126532. PMID: 34207103. PMCID: PMC8234871. PMID: 9cc32856714d4af4874eef616bd8726e. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85108078334&origin=inward.33. Singh V, Kesharwani P. 2021; Dendrimer as a promising nanocarrier for the delivery of doxorubicin as an anticancer therapeutics. J Biomater Sci Polym Ed. 32:1882–1909. DOI: 10.1080/09205063.2021.1938859. PMID: 34078252. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85111069950&origin=inward.34. Sun H, Yarovoy I, Capeling M, Cheng C. 2017; Polymers in the co-delivery of siRNA and anticancer drugs for the treatment of drug-resistant cancers. Top Curr Chem (Cham). 375:24. DOI: 10.1007/s41061-017-0113-z. PMID: 28176270. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85011843801&origin=inward.35. Sanyakamdhorn S, Agudelo D, Tajmir-Riahi HA. 2017; Review on the targeted conjugation of anticancer drugs doxorubicin and tamoxifen with synthetic polymers for drug delivery. J Biomol Struct Dyn. 35:2497–2508. DOI: 10.1080/07391102.2016.1222971. PMID: 27598545. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84986212520&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Comparative Study of the Toxicity Between carboplatin and Cisplatin in VBP Combination Chemotherapy
- A case of neoadjuvant chemotherapy with taxol / carboplatin in advanced epithelial ovarian cancer
- Phase I clinical trial of simultaneous administration of intraperitoneal carboplatin, etoposide and cytosine arabinoside for refractory ovarian carcinoma
- Ocular Complications after Injection of Intra-arterial Carboplatin in Gliomas
- Quantitative analysis of proteins related to chemoresistance to paclitaxel and carboplatin in human SiHa cervical cancer cells via iTRAQ