Nutr Res Pract.
2022 Dec;16(6):716-728. 10.4162/nrp.2022.16.6.716.
Mulberry (Morus alba L.) ethanol extract attenuates lipid metabolic disturbance and adipokine imbalance in high-fat fed rats
- Affiliations
-
- 1Department of Food and Nutrition, College of Nursing, Healthcare Sciences and Human Ecology, Dongeui University, Busan 47340, Korea
- KMID: 2536506
- DOI: http://doi.org/10.4162/nrp.2022.16.6.716
Abstract
- BACKGROUND/OBJECTIVES
An imbalanced adipokine profile in obesity increases the susceptibility to obesity-related cardiometabolic alterations, including type 2 diabetes, hypertension, dyslipidemia, and non-alcoholic fatty liver disease. The mulberry plant has been reported to have health benefits, such as hypolipidemic and hepatoprotective effects. This study examined the effects of a mulberry (Morus alba L.) fruit ethanol extract (MBEE) on dyslipidemia, liver steatosis, and adipokine imbalance in response to a high-fat diet.
MATERIALS/METHODS
Male Sprague-Dawley rats were assigned to one of 4 groups containing 6 rats each and fed either a control diet (CON), a high-fat diet (HFD), or a high-fat diet with MBEE of 150 mg/kg/day (LMB) or 300 mg/kg/day (HMB). The triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) activities were measured spectrophotometrically. The leptin, adiponectin, and plasminogen activator inhibitor-1 (PAI-1) levels were determined by an enzyme-linked immunosorbent assay.
RESULTS
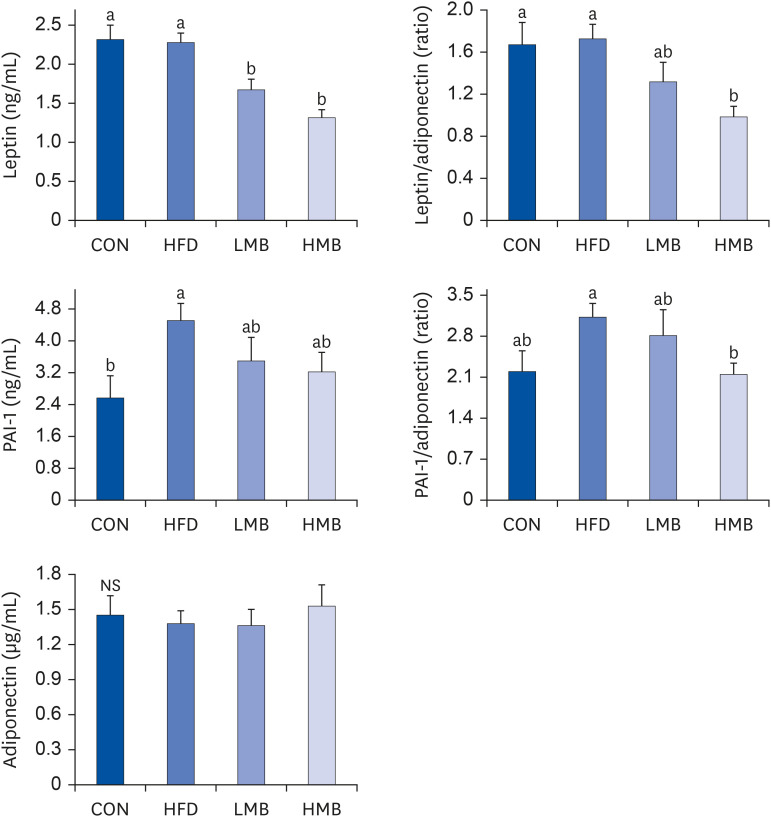
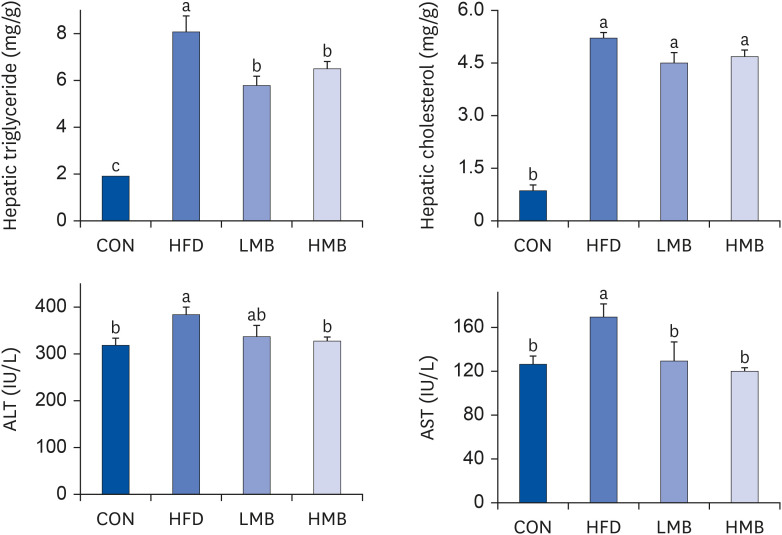
The plasma TG levels were similar in the 4 groups. Plasma cholesterol and lowdensity lipoprotein cholesterol (LDL-C) levels and TC/HDL-C ratio increased in the HFD group compared with the CON group, whereas those values decreased in the LMB group (P < 0.05), indicating that MBEE had a plasma lipid-lowering effect. HDL-C decreased in the HFD group, but MBEE did not affect the HDL-C level. The HFD rats significantly increased hepatic TG and cholesterol levels and plasma ALT and AST activities compared to the CON group. The hepatic TG level and ALT and AST activities were reduced markedly by the MBEE treatment. The HFD group showed a higher PAI-1 level, whereas MBEE treatment, especially in the HMB group, significantly reduced leptin level, and leptin/adiponectin and PAI-1/ adiponectin ratios. These findings suggest that MBEE altered the imbalance between the proand anti-inflammatory adipokines to a more anti-inflammatory state.
CONCLUSIONS
MBEE could protect against abnormal lipid metabolism and hepatic steatosis induced by a high-fat diet, lowering plasma cholesterol, LDL-C and TC/HDL-C, and hepatic TG. These findings are associated with the regulating effect of MBEE on the leptin/ adiponectin and PAI-1/adiponectin ratios.
Figure
Reference
-
1. Kishida K, Funahashi T, Shimomura I. Clinical importance of assessment of type 2 diabetes mellitus with visceral obesity. A Japanese perspective. Curr Diabetes Rev. 2012; 8:84–91. PMID: 22309596.
Article2. Calabrò P, Limongelli G, Pacileo G, Di Salvo G, Golino P, Calabrò R. The role of adiposity as a determinant of an inflammatory milieu. J Cardiovasc Med (Hagerstown). 2008; 9:450–460. PMID: 18403996.
Article3. Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Russo PE, Riegler L, Bianchi R, Crisci M, Palma GD, et al. Adipose tissue and vascular inflammation in coronary artery disease. World J Cardiol. 2014; 6:539–554. PMID: 25068015.
Article4. Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015; 36:461–470. PMID: 26022934.
Article5. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011; 11:85–97. PMID: 21252989.
Article6. Maresca F, Di Palma V, Bevilacqua M, Uccello G, Taglialatela V, Giaquinto A, Esposito G, Trimarco B, Cirillo P. Adipokines, vascular wall, and cardiovascular disease: a focused overview of the role of adipokines in the pathophysiology of cardiovascular disease. Angiology. 2015; 66:8–24. PMID: 24535638.
Article7. Adolph TE, Grander C, Grabherr F, Tilg H. Adipokines and non-alcoholic fatty liver disease: multiple interactions. Int J Mol Sci. 2017; 18:1649–1663. PMID: 28758929.
Article8. Ge F, Zhou S, Hu C, Lobdell H 4th, Berk PD. Insulin- and leptin-regulated fatty acid uptake plays a key causal role in hepatic steatosis in mice with intact leptin signaling but not in ob/ob or db/db mice. Am J Physiol Gastrointest Liver Physiol. 2010; 299:G855–G866. PMID: 20595619.9. Yanai H, Yoshida H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: mechanisms and perspectives. Int J Mol Sci. 2019; 20:1190–1214. PMID: 30857216.
Article10. López-Jaramillo P, Gómez-Arbeláez D, López-López J, López-López C, Martínez-Ortega J, Gómez-Rodríguez A, Triana-Cubillos S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Investig. 2014; 18:37–45.
Article11. Kotani K, Sakane N, Saiga K, Kurozawa Y. Leptin : adiponectin ratio as an atherosclerotic index in patients with type 2 diabetes : relationship of the index to carotid intima-media thickness. Diabetologia. 2005; 48:2684–2686. PMID: 16261311.
Article12. López-Velázquez JA, Silva-Vidal KV, Ponciano-Rodríguez G, Chávez-Tapia NC, Arrese M, Uribe M, Méndez-Sánchez N. The prevalence of nonalcoholic fatty liver disease in the Americas. Ann Hepatol. 2014; 13:166–178. PMID: 24552858.
Article13. Bradbury MW, Berk PD. Lipid metabolism in hepatic steatosis. Clin Liver Dis. 2004; 8:639–671. PMID: 15331068.
Article14. Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord. 2004; 28(Suppl 4):S12–S21. PMID: 15592481.
Article15. Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, Campbell-Thompson M, Crawford J, Shek EW, Scarpace PJ, et al. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci U S A. 2003; 100:14217–14222. PMID: 14617771.
Article16. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002; 8:1288–1295. PMID: 12368907.
Article17. Ikejima K, Honda H, Yoshikawa M, Hirose M, Kitamura T, Takei Y, Sato N. Leptin augments inflammatory and profibrogenic responses in the murine liver induced by hepatotoxic chemicals. Hepatology. 2001; 34:288–297. PMID: 11481614.
Article18. Zhang H, Ma ZF, Luo X, Li X. Effect of mulberry fruit (Morus alba L.) consumption and health outcomes: a mini-review. Antioxidants. 2018; 7:69–81. PMID: 29883416.
Article19. Ahanger MR, Ramegowda GK, Rizvi G, Dhar A, Sahaf KA. Reaction of mulberry germplasm to frost damage in Kashmir. Res J Agric Sci. 2013; 4:180–184.20. Tang CC, Huang HP, Lee YJ, Tang YH, Wang CJ. Hepatoprotective effect of mulberry water extracts on ethanol-induced liver injury via anti-inflammation and inhibition of lipogenesis in C57BL/6J mice. Food Chem Toxicol. 2013; 62:786–796. PMID: 24140469.
Article21. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502. PMID: 4337382.
Article22. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509. PMID: 13428781.
Article23. Jung UJ, Choi MS. Obesity and its metabolic implications: the Role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and non-alcoholic fatty liver disease. Int J Mol Sci. 2014; 15:6184–6223. PMID: 24733068.
Article24. Abdul Kadir NA, Rahmat A, Jaafar HZ. Protective effects of Tamarillo (Cyphomandra betacea) extract against high fat diet induced obesity in Sprague-Dawley rats. J Obes. 2015; 2015:846041. PMID: 26171246.25. Yang X, Yang L, Zheng H. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem Toxicol. 2010; 48:2374–2379. PMID: 20561945.
Article26. Song H, Lai J, Tang Q, Zheng X. Mulberry ethanol extract attenuates hepatic steatosis and insulin resistance in high-fat diet-fed mice. Nutr Res. 2016; 36:710–718. PMID: 27262537.
Article27. Kim HB, Go EJ, Ryu BR, Shin YR, Yang SJ, Back JS, Lim JD. Anti-obesity effect of mulberry anthocyanins in C57BL/6J mice. Hanguk Yakyong Changmul Hakhoe Chi. 2021; 29:317–327.
Article28. Woods SC, Seeley RJ, Rushing PA, D’Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003; 133:1081–1087. PMID: 12672923.
Article29. Saleh J, Sniderman AD, Cianflone K. Regulation of plasma fatty acid metabolism. Clin Chim Acta. 1999; 286:163–180. PMID: 10511290.
Article30. Marsh JB. Lipoprotein metabolism in obesity and diabetes: insights from stable isotope kinetic studies in humans. Nutr Rev. 2003; 61:363–375. PMID: 14677571.
Article31. Aguilera CM, Gil-Campos M, Cañete R, Gil A. Alterations in plasma and tissue lipids associated with obesity and metabolic syndrome. Clin Sci (Lond). 2008; 114:183–193. PMID: 18184112.
Article32. Hutley L, Prins JB. Fat as an endocrine organ: relationship to the metabolic syndrome. Am J Med Sci. 2005; 330:280–289. PMID: 16355012.
Article33. Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004; 89:2563–2568. PMID: 15181024.
Article34. Juhan-Vague I, Alessi MC, Mavri A, Morange PE. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost. 2003; 1:1575–1579. PMID: 12871293.
Article35. Meiliana A, Wijaya A, As’ad S. The relationship of proinflammatory and antiinflammatory adipokines in the development of metabolic syndrome in centrally obese men. Indones Biomed J. 2010; 2:118–125.
Article36. Masquio DC, de Piano A, Campos RM, Sanches PL, Carnier J, Corgosinho FC, Netto BD, Carvalho-Ferreira JP, Oyama LM, Nascimento CM, et al. The role of multicomponent therapy in the metabolic syndrome, inflammation and cardiovascular risk in obese adolescents. Br J Nutr. 2015; 113:1920–1930. PMID: 25907896.
Article37. Mikami K, Endo T, Sawada N, Igarashi G, Kimura M, Hasegawa T, Iino C, Tomita H, Sawada K, Nakaji S, et al. Leptin/adiponectin ratio correlates with hepatic steatosis but not arterial stiffness in nonalcoholic fatty liver disease in Japanese population. Cytokine. 2020; 126:154927. PMID: 31756645.
Article38. Adejumo EN, Adejumo OA, Azenabor A, Ekun AO, Enitan SS, Adebola OK, Ogundahunsi OA. Leptin: adiponectin ratio discriminated the risk of metabolic syndrome better than adiponectin and leptin in Southwest Nigeria. Diabetes Metab Syndr. 2019; 13:1845–1849. PMID: 31235104.
Article39. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018; 24:908–922. PMID: 29967350.
Article40. Packard CJ, Boren J, Taskinen MR. Causes and consequences of hypertriglyceridemia. Front Endocrinol (Lausanne). 2020; 11:252–266. PMID: 32477261.
Article41. Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol. 2017; 8:1–8. PMID: 29357123.42. Farrell NJ, Norris GH, Ryan J, Porter CM, Jiang C, Blesso CN. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. Br J Nutr. 2015; 114:1123–1131. PMID: 26314315.
Article43. Marques V, Afonso MB, Bierig N, Duarte-Ramos F, Santos-Laso Á, Jimenez-Agüero R, Eizaguirre E, Bujanda L, Pareja MJ, Luís R, et al. Adiponectin, leptin, and IGF-1 are useful diagnostic and stratification biomarkers of NAFLD. Front Med (Lausanne). 2021; 8:683250. PMID: 34249975.
Article44. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017; 18:1321–1337. PMID: 28635626.
Article45. Bril F, Barb D, Portillo-Sanchez P, Biernacki D, Lomonaco R, Suman A, Weber MH, Budd JT, Lupi ME, Cusi K. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017; 65:1132–1144. PMID: 27981615.
Article46. Chang JJ, Hsu MJ, Huang HP, Chung DJ, Chang YC, Wang CJ. Mulberry anthocyanins inhibit oleic acid induced lipid accumulation by reduction of lipogenesis and promotion of hepatic lipid clearance. J Agric Food Chem. 2013; 61:6069–6076. PMID: 23731091.
Article47. Jiang Y, Dai M, Nie WJ, Yang XR, Zeng XC. Effects of the ethanol extract of black mulberry (Morus nigra L.) fruit on experimental atherosclerosis in rats. J Ethnopharmacol. 2017; 200:228–235. PMID: 28242382.
Article48. Chen CC, Liu LK, Hsu JD, Huang HP, Yang MY, Wang CJ. Mulberry extract inhibits the development of atherosclerosis in cholesterol-fed rabbits. Food Chem. 2005; 91:601–607.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amelioration of metabolic disturbances and adipokine dysregulation by mugwort (Artemisia princeps P.) extract in high-fat diet-induced obese rats
- Inhibitory effect of water-soluble mulberry leaf extract on hepatic lipid accumulation in high-fat diet-fed rats via modulation of hepatic microRNA-221/222 expression and inflammation
- Effect of Eisenia Bicyclis and Its Pill on Serum Lipid Status in Rats Fed High Fat Diet
- Morus Nigra Extract Attenuates Cognition Impairment and GABAergic Interneuron Degeneration in Aged Rat Brain
- Effects of Various Mulberry Products on the Blood Glucose and Lipid Status of Streptozotocin-induced Diabetic Rats