Diabetes Metab J.
2022 Nov;46(6):901-911. 10.4093/dmj.2021.0314.
Effect of the Glucagon-Like Peptide-1 Receptor Agonists on Autonomic Function in Subjects with Diabetes: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Unit of Endocrinology, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Modena, Italy
- 2Unit of Endocrinology, Department of Medical Specialties, Baggiovara Hospital, University Hospital of Modena, Modena, Italy
- KMID: 2536148
- DOI: http://doi.org/10.4093/dmj.2021.0314
Abstract
- Background
In addition to the metabolic effects in diabetes, glucagon-like peptide 1 receptor (GLP-1R) agonists lead to a small but substantial increase in heart rate (HR). However, the GLP-1R actions on the autonomic nervous system (ANS) in diabetes remain debated. Therefore, this meta-analysis evaluates the effect of GLP-1R agonist on measures of ANS function in diabetes.
Methods
According to the Cochrane Collaboration and Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement, we conducted a meta-analysis considering clinical trials in which the autonomic function was evaluated in diabetic subjects chronically treated with GLP-1R agonists. The outcomes were the change of ANS function measured by heart rate variability (HRV) and cardiac autonomic reflex tests (CARTs).
Results
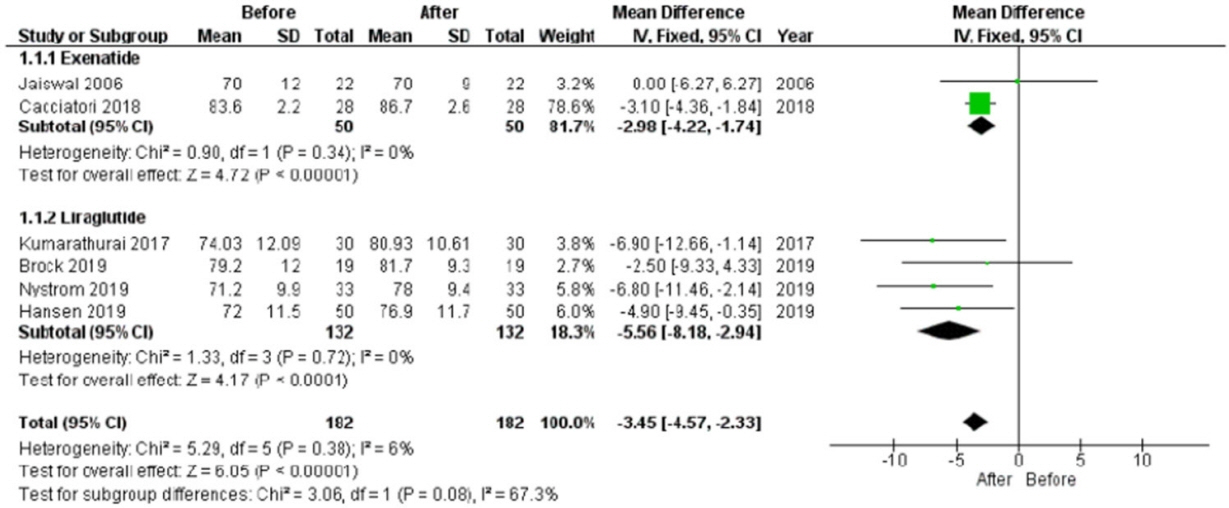
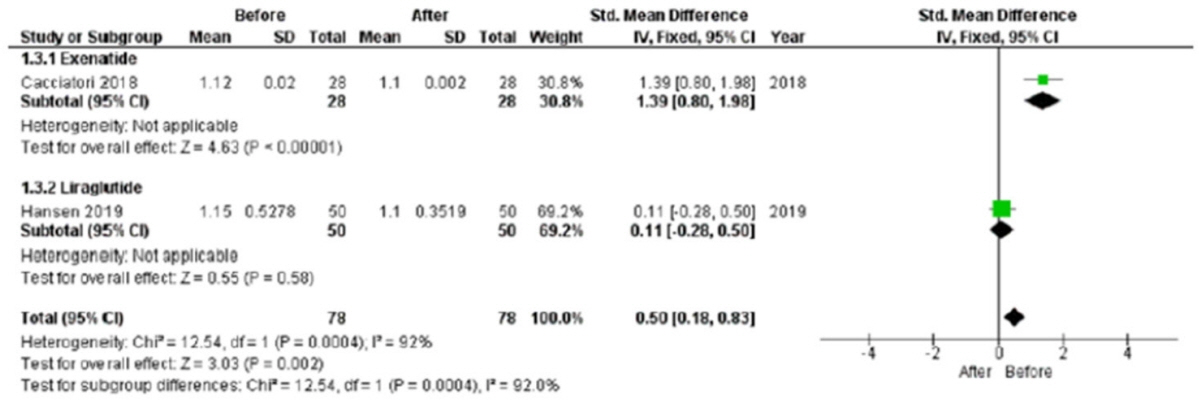
In the studies enrolled, HR significantly increased after treatment (P<0.001), whereas low frequency/high frequency ratio did not differ (P=0.410); no changes in other measures of HRV were detected. Considering CARTs, only the 30:15 value derived from lying-to-standing test was significantly lower after treatment (P=0.002), but only two studies reported this measurement. No differences in other CARTs outcome were observed.
Conclusion
The meta-analysis confirms the HR increase but seems to exclude an alteration of the sympatho-vagal balance due to chronic treatment with GLP-1R agonists in diabetes, considering the available measures of ANS function.
Keyword
Figure
Reference
-
1. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010; 33:2285–93.
Article2. Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011; 27:639–53.
Article3. Neil HA, Thompson AV, John S, McCarthy ST, Mann JI. Diabetic autonomic neuropathy: the prevalence of impaired heart rate variability in a geographically defined population. Diabet Med. 1989; 6:20–4.
Article4. Ziegler D, Gries FA, Spuler M, Lessmann F. The epidemiology of diabetic neuropathy. DiaCAN Multicenter Study Group. Diabet Med. 1993; 10 Suppl 2:82S–86S.
Article5. Zoppini G, Cacciatori V, Raimondo D, Gemma M, Trombetta M, Dauriz M, et al. Prevalence of cardiovascular autonomic neuropathy in a cohort of patients with newly diagnosed type 2 diabetes: the Verona Newly Diagnosed Type 2 Diabetes Study (VNDS). Diabetes Care. 2015; 38:1487–93.
Article6. Williams SM, Eleftheriadou A, Alam U, Cuthbertson DJ, Wilding JP. Correction to: Cardiac autonomic neuropathy in obesity, metabolic syndrome and prediabetes: a narrative review. Diabetes Ther. 2019; 10:2023.
Article7. Spallone V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J. 2019; 43:3–30.
Article8. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017; 40:136–54.
Article9. Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007; 115:387–97.
Article10. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018; 27:740–56.
Article11. Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010; 159:612–9.
Article12. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016; 375:311–22.13. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015; 373:2247–57.
Article14. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016; 375:1834–44.
Article15. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017; 377:1228–39.
Article16. Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018; 392:1519–29.17. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019; 394:121–30.18. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019; 381:841–51.
Article19. Katout M, Zhu H, Rutsky J, Shah P, Brook RD, Zhong J, et al. Effect of GLP-1 mimetics on blood pressure and relationship to weight loss and glycemia lowering: results of a systematic meta-analysis and meta-regression. Am J Hypertens. 2014; 27:130–9.
Article20. Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004; 287:E1209–15.21. Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab. 2004; 89:3055–61.
Article22. Sun F, Wu S, Guo S, Yu K, Yang Z, Li L, et al. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Res Clin Pract. 2015; 110:26–37.
Article23. England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, et al. Practice parameter: evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review): report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009; 72:177–84.
Article24. Bernardi L, Spallone V, Stevens M, Hilsted J, Frontoni S, PopBusui R, et al. Methods of investigation for cardiac autonomic dysfunction in human research studies. Diabetes Metab Res Rev. 2011; 27:654–64.25. Brock C, Hansen CS, Karmisholt J, Moller HJ, Juhl A, Farmer AD, et al. Liraglutide treatment reduced interleukin-6 in adults with type 1 diabetes but did not improve established autonomic or polyneuropathy. Br J Clin Pharmacol. 2019; 85:2512–23.
Article26. Cacciatori V, Zoppini G, Bellavere F, Rigolon R, Thomaseth K, Pichiri I, et al. Long-acting GLP-1 receptor agonist exenatide influence on the autonomic cardiac sympatho-vagal balance. J Endocr Soc. 2017; 2:53–62.
Article27. Hansen CS, Frandsen CS, Fleischer J, Vistisen D, Holst JJ, Tarnow L, et al. Liraglutide-induced weight loss may be affected by autonomic regulation in type 1 diabetes. Front Endocrinol (Lausanne). 2019; 10:242.
Article28. Jaiswal M, Martin CL, Brown MB, Callaghan B, Albers JW, Feldman EL, et al. Effects of exenatide on measures of diabetic neuropathy in subjects with type 2 diabetes: results from an 18-month proof-of-concept open-label randomized study. J Diabetes Complications. 2015; 29:1287–94.
Article29. Kumarathurai P, Anholm C, Fabricius-Bjerre A, Nielsen OW, Kristiansen O, Madsbad S, et al. Effects of the glucagon-like peptide-1 receptor agonist liraglutide on 24-h ambulatory blood pressure in patients with type 2 diabetes and stable coronary artery disease: a randomized, double-blind, placebo-controlled, crossover study. J Hypertens. 2017; 35:1070–8.30. Nystrom T, Santos-Pardo I, Fang X, Cao Y, Hedberg F, Jendle J. Heart rate variability in type 2 diabetic subjects randomized to liraglutide or glimepiride treatment, both in combination with metformin: a randomized, open, parallel-group study. Endocrinol Diabetes Metab. 2019; 2:e00058.31. Kumarathurai P, Anholm C, Larsen BS, Olsen RH, Madsbad S, Kristiansen O, et al. Effects of liraglutide on heart rate and heart rate variability: a randomized, double-blind, placebocontrolled crossover study. Diabetes Care. 2017; 40:117–24.32. Goldstein DS, Bentho O, Park MY, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol. 2011; 96:1255–61.
Article33. Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol. 2013; 4:26.
Article34. Baggio LL, Ussher JR, McLean BA, Cao X, Kabir MG, Mulvihill EE, et al. The autonomic nervous system and cardiac GLP1 receptors control heart rate in mice. Mol Metab. 2017; 6:1339–49.
Article35. Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002; 110:43–52.
Article36. Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014; 63:1224–33.
Article37. Pyke C, Heller RS, Kirk RK, Orskov C, Reedtz-Runge S, Kaastrup P, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014; 155:1280–90.38. Berkelaar M, Eekhoff EM, Simonis-Bik AM, Boomsma DI, Diamant M, Ijzerman RG, et al. Effects of induced hyperinsulinaemia with and without hyperglycaemia on measures of cardiac vagal control. Diabetologia. 2013; 56:1436–43.
Article39. Valensi P, Chiheb S, Fysekidis M. Insulin- and glucagon-like peptide-1-induced changes in heart rate and vagosympathetic activity: why they matter. Diabetologia. 2013; 56:1196–200.
Article40. Bharucha AE, Charkoudian N, Andrews CN, Camilleri M, Sletten D, Zinsmeister AR, et al. Effects of glucagon-like peptide-1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol. 2008; 295:R874–80.
Article41. Smits MM, Muskiet MH, Tonneijck L, Hoekstra T, Kramer MH, Diamant M, et al. Exenatide acutely increases heart rate in parallel with augmented sympathetic nervous system activation in healthy overweight males. Br J Clin Pharmacol. 2016; 81:613–20.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glucagon-like peptide-1 and glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes
- Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association
- The Global Shortage of Glucagon-Like Peptide-1 Receptor Agonists
- The Road towards Triple Agonists: Glucagon-Like Peptide 1, Glucose-Dependent Insulinotropic Polypeptide and Glucagon Receptor - An Update
- The Effects of Hypoglycemic Agents on Non-alcoholic Fatty Liver Disease: Focused on Sodium-Glucose Cotransporter 2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists