Ann Rehabil Med.
2022 Oct;46(5):263-273. 10.5535/arm.22014.
Effects of Intensive Exercise on Cognitive Dysfunction in Patients With Pure Cerebellar Degeneration: A Single-Arm Pilot Study
- Affiliations
-
- 1Department of Rehabilitation, Kumamoto Southern Regional Hospital, Kumamoto, Japan
- 2Department of Neurology, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Japan
- 3Department of Neurology, Kumamoto Southern Regional Hospital, Kumamoto, Japan
- 4Department of Rehabilitation, Kumamoto Health Science University, Kumamoto, Japan
- KMID: 2535744
- DOI: http://doi.org/10.5535/arm.22014
Abstract
Objective
To clarify the profile of cognitive dysfunction and the effects of intensive exercise in spinocerebellar degeneration (SCD).
Methods
We enrolled 60 healthy controls and 16 patients with purely cerebellar type SCD without gait disturbance or organic changes other than cerebellar changes. To assess cognitive function, we evaluated the participants using the Mini-Mental State Examination (MMSE), Frontal Assessment Battery (FAB), and Montreal Cognitive Assessment-Japanese (MoCA-J) at admission and after intensive exercise.
Results
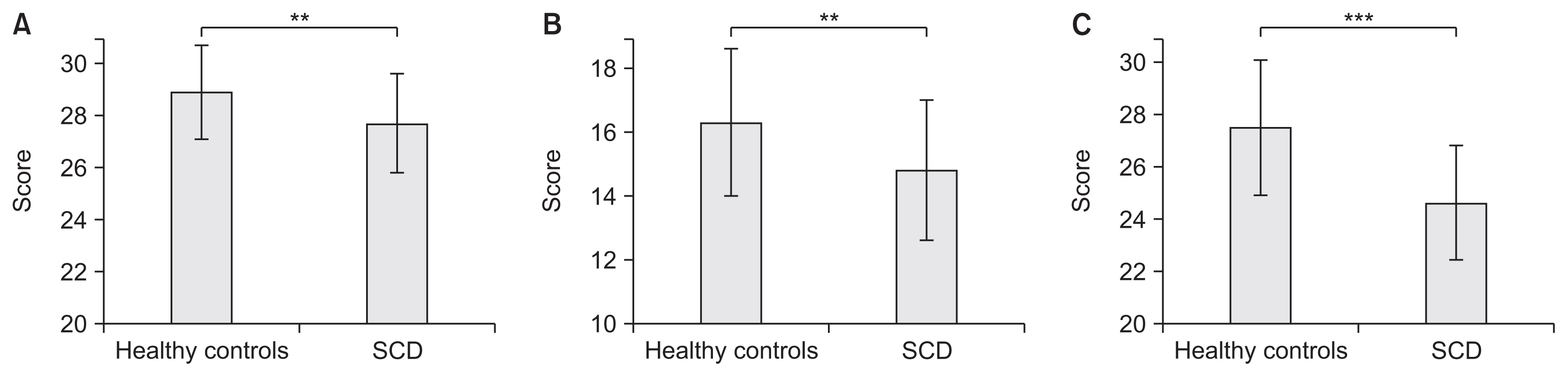
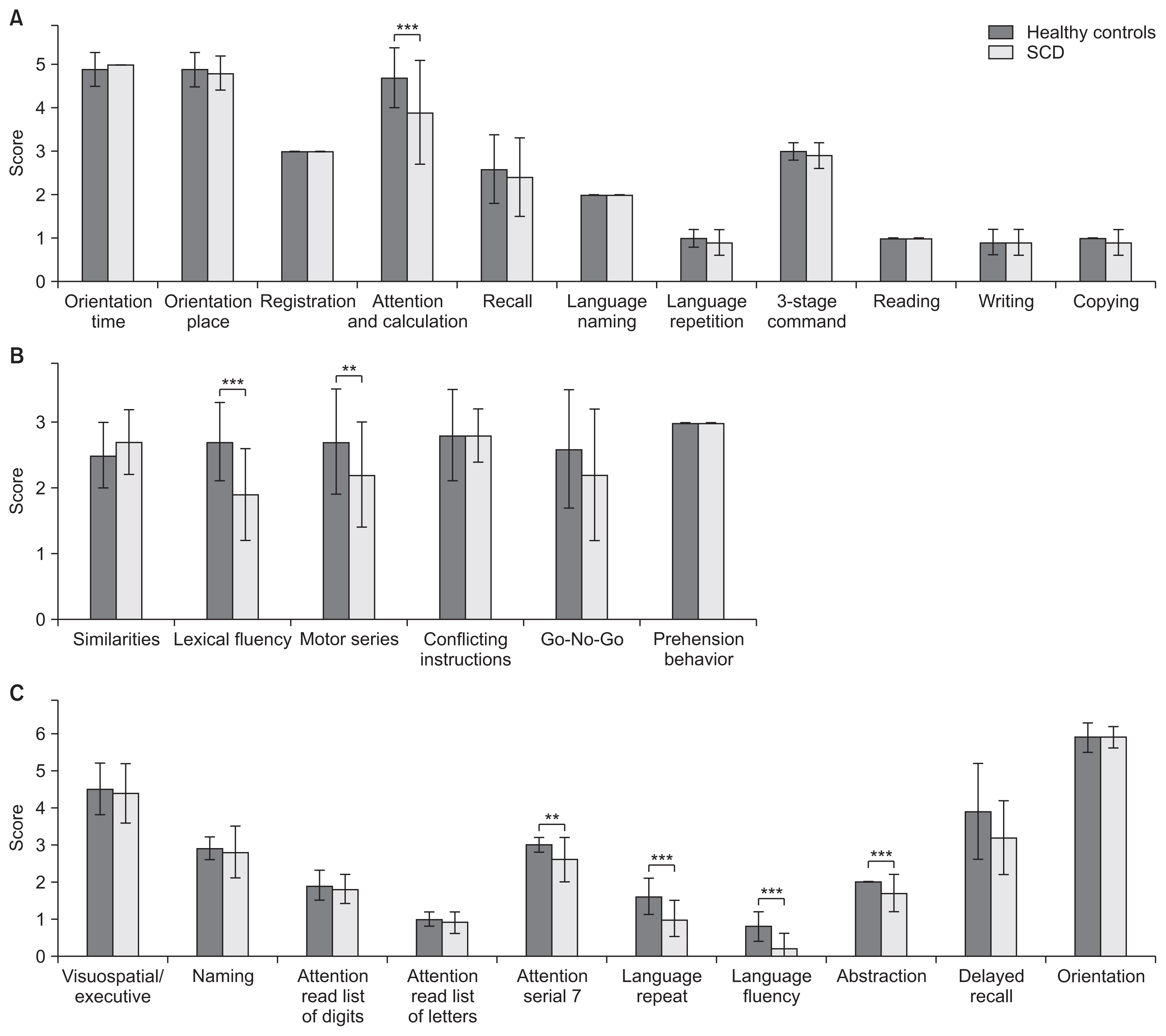
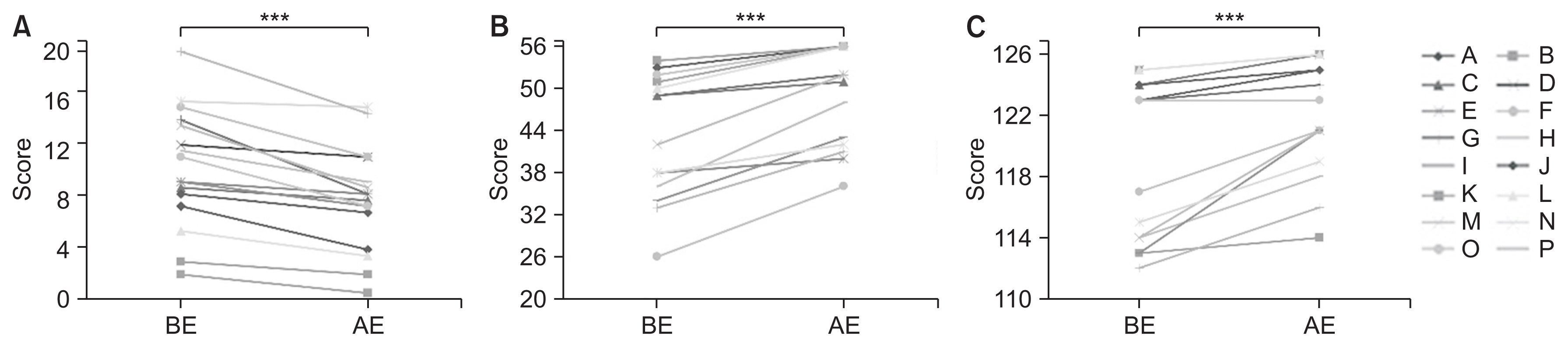
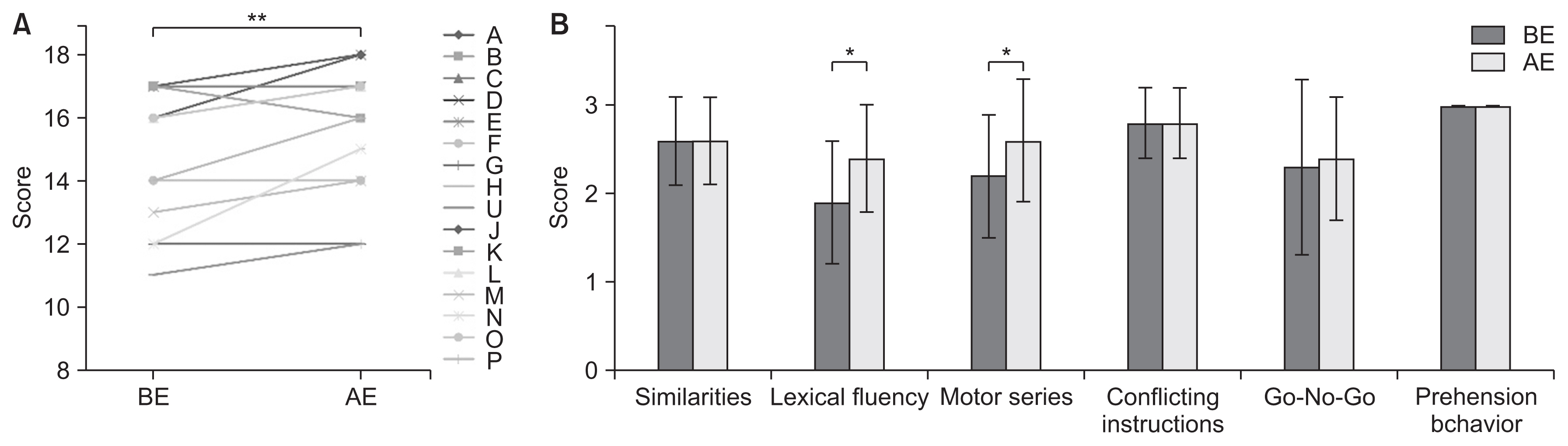
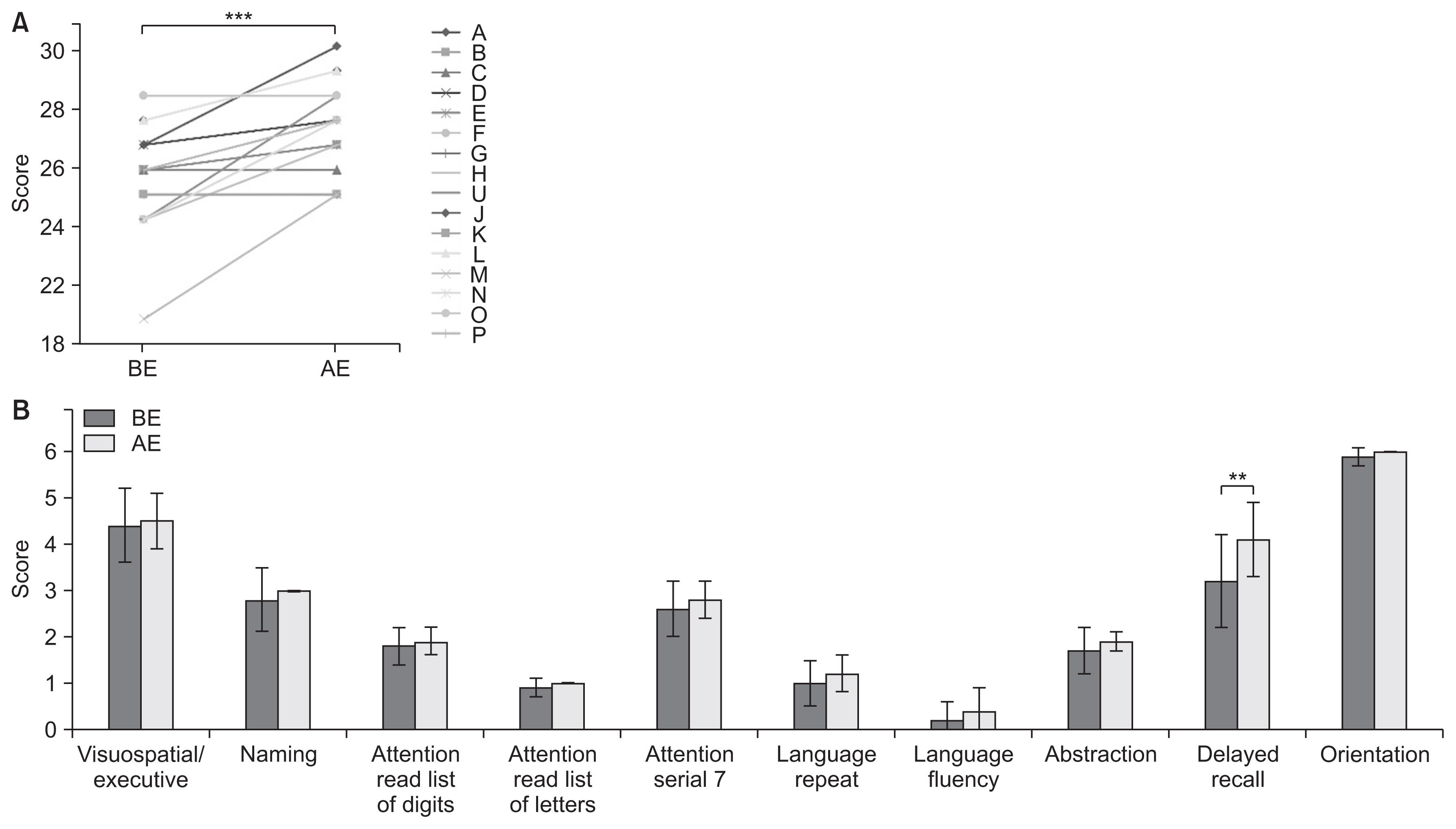
Compared to the controls, SCD patients showed significant cognitive decline. As a result of intensive exercise, significant improvements in motor and cognitive functions were observed: the MMSE score improved from 27.7±1.9 to 29.0±1.3 points (p<0.001); the FAB score improved from 14.8±2.2 to 15.8±2.0 points (p=0.002); and the MoCA-J score improved from 24.6±2.2 to 26.7±1.9 points (p<0.001). For sub-scores, significant improvements were noted in serial 7, lexical fluency, motor series, and delayed recall.
Conclusion
Our study indicates that intensive exercise can be effective not only for motor dysfunction but also for cognitive dysfunction (Clinical Trial Registration No. UMIN-CTR: UMIN000040079).
Figure
Reference
-
1. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998; 121(Pt 4):561–79.
Article2. Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011; 106:2322–45.
Article3. Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009; 44:489–501.
Article4. EKH , Chen SH, Ho MH, Desmond JE. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp. 2014; 35:593–615.
Article5. Burk K, Globas C, Bosch S, Graber S, Abele M, Brice A, et al. Cognitive deficits in spinocerebellar ataxia 2. Brain. 1999; 122(Pt 4):769–77.
Article6. Burk K, Bosch S, Globas C, Zuhlke C, Daum I, Klockgether T, et al. Executive dysfunction in spinocerebellar ataxia type 1. Eur Neurol. 2001; 46:43–8.
Article7. Kawai Y, Takeda A, Abe Y, Washimi Y, Tanaka F, Sobue G. Cognitive impairments in Machado-Joseph disease. Arch Neurol. 2004; 61:1757–60.
Article8. Suenaga M, Kawai Y, Watanabe H, Atsuta N, Ito M, Tanaka F, et al. Cognitive impairment in spinocerebellar ataxia type 6. J Neurol Neurosurg Psychiatry. 2008; 79:496–9.
Article9. Cooper FE, Grube M, Elsegood KJ, Welch JL, Kelly TP, Chinnery PF, et al. The contribution of the cerebellum to cognition in spinocerebellar ataxia type 6. Behav Neurol. 2010; 23:3–15.
Article10. Kawahara Y, Ikeda Y, Deguchi K, Kurata T, Hishikawa N, Sato K, et al. Simultaneous assessment of cognitive and affective functions in multiple system atrophy and cortical cerebellar atrophy in relation to computerized touch-panel screening tests. J Neurol Sci. 2015; 351:24–30.
Article11. Tamura I, Takei A, Hamada S, Nonaka M, Kurosaki Y, Moriwaka F. Cognitive dysfunction in patients with spinocerebellar ataxia type 6. J Neurol. 2017; 264:260–7.
Article12. Ilg W, Synofzik M, Brotz D, Burkard S, Giese MA, Schols L. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology. 2009; 73:1823–30.
Article13. Miyai I, Ito M, Hattori N, Mihara M, Hatakenaka M, Yagura H, et al. Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases. Neurorehabil Neural Repair. 2012; 26:515–22.
Article14. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53:695–9.
Article15. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982; 14:377–81.
Article16. Schlosser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, et al. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatry. 1998; 64:492–8.
Article17. Rentiya ZS, Jung BC, Bae J, Liszewski CM, Fishman A, Du AX, et al. Selective patterns of cognitive impairment in spinocerebellar ataxia type 6 and idiopathic late-onset cerebellar ataxia. Arch Clin Neuropsychol. 2018; 33:427–36.
Article18. Bristow T, Jih CS, Slabich A, Gunn J. Standardization and adult norms for the sequential subtracting tasks of serial 3’s and 7’s. Appl Neuropsychol Adult. 2016; 23:372–8.
Article19. Moriarty A, Cook A, Hunt H, Adams ME, Cipolotti L, Giunti P. A longitudinal investigation into cognition and disease progression in spinocerebellar ataxia types 1, 2, 3, 6, and 7. Orphanet J Rare Dis. 2016; 11:82.
Article20. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000; 55:1621–6.
Article21. Taskiran-Sag A, Uzuncakmak Uyanik H, Uyanik SA, Oztekin N. Prospective investigation of cerebellar cognitive affective syndrome in a previously non-demented population of acute cerebellar stroke. J Stroke Cerebrovasc Dis. 2020; 29:104923.
Article22. Burk K, Globas C, Bosch S, Klockgether T, Zuhlke C, Daum I, et al. Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. J Neurol. 2003; 250:207–11.
Article23. Justus T. The cerebellum and English grammatical morphology: evidence from production, comprehension, and grammaticality judgments. J Cogn Neurosci. 2004; 16:1115–30.
Article24. Ronning C, Sundet K, Due-Tonnessen B, Lundar T, Helseth E. Persistent cognitive dysfunction secondary to cerebellar injury in patients treated for posterior fossa tumors in childhood. Pediatr Neurosurg. 2005; 41:15–21.
Article25. Orsi L, D’Agata F, Caroppo P, Franco A, Caglio MM, Avidano F, et al. Neuropsychological picture of 33 spinocerebellar ataxia cases. J Clin Exp Neuropsychol. 2011; 33:315–25.
Article26. Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain. 2018; 141:248–70.
Article27. Kawai Y, Suenaga M, Watanabe H, Ito M, Kato K, Kato T, et al. Prefrontal hypoperfusion and cognitive dysfunction correlates in spinocerebellar ataxia type 6. J Neurol Sci. 2008; 271:68–74.
Article28. Valenzuela PL, Castillo-Garcia A, Morales JS, de la Villa P, Hampel H, Emanuele E, et al. Exercise benefits on Alzheimer’s disease: state-of-the-science. Ageing Res Rev. 2020; 62:101108.
Article29. Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013; 12:716–26.
Article30. Petzinger GM, Holschneider DP, Fisher BE, McEwen S, Kintz N, Halliday M, et al. The effects of exercise on dopamine neurotransmission in Parkinson’s disease: targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast. 2015; 1:29–39.
Article31. Avenali M, Picascia M, Minafra B, Tassorelli C, Sinforiani E, Bernini S. Intensive physical therapy mitigates cognitive decline in people with Parkinson’s disease. J Alzheimers Dis Parkinsonism. 2019; 9:475.32. Cendelin J, Korelusova I, Vozeh F. Preliminary study of the effect of repeated motor training on spatial learning ability in adult lurcher mutant mice. Prague Med Rep. 2007; 108:49–56.33. Burciu RG, Fritsche N, Granert O, Schmitz L, Sponemann N, Konczak J, et al. Brain changes associated with postural training in patients with cerebellar degeneration: a voxel-based morphometry study. J Neurosci. 2013; 33:4594–604.
Article34. Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012; 35:73–89.
Article35. Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012; 59:1560–70.
Article36. Kansal K, Yang Z, Fishman AM, Sair HI, Ying SH, Jedynak BM, et al. Structural cerebellar correlates of cognitive and motor dysfunctions in cerebellar degeneration. Brain. 2017; 140:707–20.
Article37. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007; 30:464–72.
Article38. De Miguel Z, Khoury N, Betley MJ, Lehallier B, Willoughby D, Olsson N, et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature. 2021; 600:494–9.
Article39. Lee JM, Kim CJ, Park JM, Song MK, Kim YJ. Effect of treadmill exercise on spatial navigation impairment associated with cerebellar Purkinje cell loss following chronic cerebral hypoperfusion. Mol Med Rep. 2018; 17:8121–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of HIV Infection with Progressive Cerebellar Dysfunction as Initial Manifestation
- Paraneoplastic Cerebellar Degeneration Presented as Acute Vertigo
- Cerebellar Degeneration Associated with Sjogren's Syndrome
- Effective Approach to Improving Cognitive Function in the Elderly: Focused on Cognitive-exercise Combination Program
- Effects of Phytoncide Inhalation on Stroop Task Performance in Patients with Mild Cognitive Impairment: An fNIRS Pilot Study