Acute Crit Care.
2022 Aug;37(3):415-428. 10.4266/acc.2021.01508.
Cytokine profiles in intensive care unit delirium
- Affiliations
-
- 1Mayo Clinic School of Graduate Medical Education, Department of Internal Medicine, Mayo Clinic, Rochester, MN, USA
- 2Division of Psychiatry, Department of Neurology, Mayo Clinic Florida, Jacksonville, FL, USA
- 3Division of Gastroenterology and Hepatology, Mayo Clinic Arizona, Phoenix, AZ, USA
- 4Department of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA
- 5Department of Critical Care Medicine, Mayo Clinic Arizona, Phoenix, AZ, USA
- 6Division of Pulmonary Medicine, Mayo Clinic Arizona, Phoenix, AZ, USA
- KMID: 2535306
- DOI: http://doi.org/10.4266/acc.2021.01508
Abstract
- Background
Neuroinflammation causing disruption of the blood-brain barrier and immune cell extravasation into the brain parenchyma may cause delirium; however, knowledge of the exact pathophysiologic mechanism remains incomplete. The purpose of our study was to determine whether cytokine profiles differ depending on whether delirium occurs in the setting of sepsis, coronavirus disease 2019 (COVID-19), or recent surgery.
Methods
This prospective observational cohort study involved 119 critically ill patients admitted to a multidisciplinary intensive care unit (ICU) during 2019 and 2020. Delirium was identified using the validated confusion assessment method for the ICU. Multiple delirium risk factors were collected daily including clinical characteristics, hospital course, lab values, vital signs, surgical exposure, drug exposure, and COVID-19 characteristics. Serums samples were collected within 12 hours of ICU admission and cytokine levels were measured.
Results
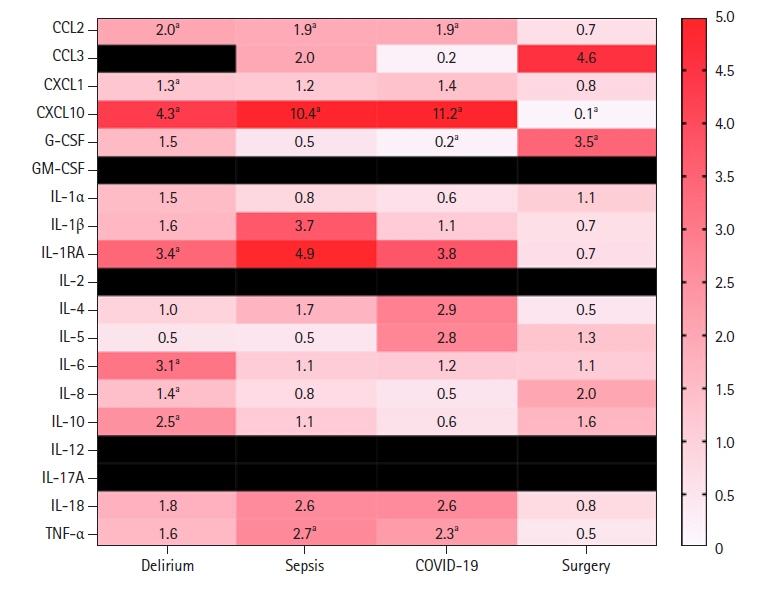
The following proinflammatory cytokines were elevated in our delirium population: tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-18, C-C motif ligand (CCL) 2, CCL3, C-X-C motif chemokine ligand (CXCL)1, CXCL10, IL-8, IL-1 receptor antagonist, and IL-10. Analysis of relative cytokine levels in those patients that developed delirium in the setting of sepsis, COVID-19, and recent surgery showed elevations of CCL2, CXCL10, and TNF-α in both the sepsis and COVID-19 group in comparison to the postsurgical population. In the postsurgical group, granulocyte colony-stimulating factor was elevated and CXCL10 was decreased relative to the opposing groups.
Conclusions
We identify several cytokines and precipitating factors known to be associated with delirium. However, our study suggests that the cytokine profile associated with delirium is variable and contingent upon delirium precipitating factors.
Figure
Reference
-
1. Oldham MA, Flaherty JH, Maldonado JR. Refining delirium: a transtheoretical model of delirium disorder with preliminary neurophysiologic subtypes. Am J Geriatr Psychiatry. 2018; 26:913–24.
Article2. Wilson JE, Mart MF, Cunningham C, Shehabi Y, Girard TD, MacLullich AM, et al. Delirium. Nat Rev Dis Primers. 2020; 6:90.
Article3. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017; 33:461–519.4. Mattison ML. Delirium. Ann Intern Med. 2020; 173:ITC49-64.
Article5. Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med. 2014; 370:444–54.
Article6. Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017; 318:1161–74.7. Kealy J, Murray C, Griffin EW, Lopez-Rodriguez AB, Healy D, Tortorelli LS, et al. Acute inflammation alters brain energy metabolism in mice and humans: role in suppressed spontaneous activity, impaired cognition, and delirium. J Neurosci. 2020; 40:5681–96.
Article8. Maldonado JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. 2018; 33:1428–57.
Article9. de Rooij SE, van Munster BC, Korevaar JC, Levi M. Cytokines and acute phase response in delirium. J Psychosom Res. 2007; 62:521–5.
Article10. Skrede K, Wyller TB, Watne LO, Seljeflot I, Juliebø V. Is there a role for monocyte chemoattractant protein-1 in delirium?: novel observations in elderly hip fracture patients. BMC Res Notes. 2015; 8:186.
Article11. Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020; 125:492–504.
Article12. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017; 377:1456–66.
Article13. Yang T, Velagapudi R, Terrando N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol. 2020; 21:1319–26.
Article14. Honarmand K, Lalli RS, Priestap F, Chen JL, McIntyre CW, Owen AM, et al. Natural history of cognitive impairment in critical illness survivors: a systematic review. Am J Respir Crit Care Med. 2020; 202:193–201.
Article15. Wu YC, Tseng PT, Tu YK, Hsu CY, Liang CS, Yeh TC, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019; 76:526–35.
Article16. Lauretani F, Bellelli G, Pelà G, Morganti S, Tagliaferri S, Maggio M. Treatment of delirium in older persons: what we should not do! Int J Mol Sci. 2020; 21:2397.
Article17. Munster BC, Aronica E, Zwinderman AH, Eikelenboom P, Cunningham C, Rooij SE. Neuroinflammation in delirium: a postmortem case-control study. Rejuvenation Res. 2011; 14:615–22.
Article18. Burkhart CS, Dell-Kuster S, Siegemund M, Pargger H, Marsch S, Strebel SP, et al. Effect of n-3 fatty acids on markers of brain injury and incidence of sepsis-associated delirium in septic patients. Acta Anaesthesiol Scand. 2014; 58:689–700.
Article19. van den Boogaard M, Kox M, Quinn KL, van Achterberg T, van der Hoeven JG, Schoonhoven L, et al. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care. 2011; 15:R297.
Article20. Cape E, Hall RJ, van Munster BC, de Vries A, Howie SE, Pearson A, et al. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1β in delirium after hip fracture. J Psychosom Res. 2014; 77:219–25.
Article21. Subramaniyan S, Terrando N. Neuroinflammation and perioperative neurocognitive disorders. Anesth Analg. 2019; 128:781–8.
Article22. Andonegui G, Zelinski EL, Schubert CL, Knight D, Craig LA, Winston BW, et al. Targeting inflammatory monocytes in sepsis-associated encephalopathy and long-term cognitive impairment. JCI Insight. 2018; 3:e99364.
Article23. Beloosesky Y, Hendel D, Weiss A, Hershkovitz A, Grinblat J, Pirotsky A, et al. Cytokines and C-reactive protein production in hip-fracture-operated elderly patients. J Gerontol A Biol Sci Med Sci. 2007; 62:420–6.
Article24. Atterton B, Paulino MC, Povoa P, Martin-Loeches I. Sepsis associated delirium. Medicina (Kaunas). 2020; 56:240.
Article25. Vasunilashorn SM, Ngo L, Inouye SK, Libermann TA, Jones RN, Alsop DC, et al. Cytokines and postoperative delirium in older patients undergoing major elective surgery. J Gerontol A Biol Sci Med Sci. 2015; 70:1289–95.
Article26. Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013; 369:1306–16.
Article27. Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010; 119:737–54.
Article28. Guo Y, Li Y, Zhang Y, Fang S, Xu X, Zhao A, et al. Post-operative delirium associated with metabolic alterations following hemi-arthroplasty in older patients. Age Ageing. 2019; 49:88–95.
Article29. Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008; 65:229–38.
Article30. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013; 21:1190–222.31. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018; 128:2657–69.
Article32. Dabrowski W, Siwicka-Gieroba D, Gasinska-Blotniak M, Zaid S, Jezierska M, Pakulski C, et al. Pathomechanisms of non-traumatic acute brain injury in critically ill patients. Medicina (Kaunas). 2020; 56:469.
Article33. Watt CL, Momoli F, Ansari MT, Sikora L, Bush SH, Hosie A, et al. The incidence and prevalence of delirium across palliative care settings: a systematic review. Palliat Med. 2019; 33:865–77.
Article34. Mukaetova-Ladinska EB, Kronenberg G, Raha-Chowdhury R. COVID-19 and neurocognitive disorders. Curr Opin Psychiatry. 2021; 34:149–56.
Article35. Hawkins M, Sockalingam S, Bonato S, Rajaratnam T, Ravindran M, Gosse P, et al. A rapid review of the pathoetiology, presentation, and management of delirium in adults with COVID-19. J Psychosom Res. 2021; 141:110350.
Article36. Najjar S, Najjar A, Chong DJ, Pramanik BK, Kirsch C, Kuzniecky RI, et al. Central nervous system complications associated with SARS-CoV-2 infection: integrative concepts of pathophysiology and case reports. J Neuroinflammation. 2020; 17:231.
Article37. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–10.
Article38. Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001; 286:2703–10.39. Intensive Care Network. Apache IV score [Internet]. Intensive Care Network;2021. [cited 2021 Apr 22]. Available from: https://intensivecarenetwork.com/Calculators/Files/Apache4.html.40. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: on behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996; 22:707–10.
Article41. de Oliveira C, Khatua B, Noel P, Kostenko S, Bag A, Balakrishnan B, et al. Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation. J Clin Invest. 2020; 130:1931–47.
Article42. Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011; 3:107ra110.
Article43. Moraes CA, Zaverucha-do-Valle C, Fleurance R, Sharshar T, Bozza FA, d’Avila JC. Neuroinflammation in sepsis: molecular pathways of microglia activation. Pharmaceuticals (Basel). 2021; 14:416.
Article44. Galea I, Perry VH. The blood-brain interface: a culture change. Brain Behav Immun. 2018; 68:11–6.
Article45. Alexander JJ, Jacob A, Cunningham P, Hensley L, Quigg RJ. TNF is a key mediator of septic encephalopathy acting through its receptor, TNF receptor-1. Neurochem Int. 2008; 52:447–56.
Article46. Smyth LC, Rustenhoven J, Park TI, Schweder P, Jansson D, Heppner PA, et al. Unique and shared inflammatory profiles of human brain endothelia and pericytes. J Neuroinflammation. 2018; 15:138.
Article47. Banks WA, Dohi K, Hansen K, Thompson HJ. Assessing blood granulocyte colony-stimulating factor as a potential biomarker of acute traumatic brain injury in mice and humans. Brain Behav Immun. 2016; 52:81–7.
Article48. Li L, McBride DW, Doycheva D, Dixon BJ, Krafft PR, Zhang JH, et al. G-CSF attenuates neuroinflammation and stabilizes the blood-brain barrier via the PI3K/Akt/GSK-3β signaling pathway following neonatal hypoxia-ischemia in rats. Exp Neurol. 2015; 272:135–44.
Article49. Chui R, Dorovini-Zis K. Regulation of CCL2 and CCL3 expression in human brain endothelial cells by cytokines and lipopolysaccharide. J Neuroinflammation. 2010; 7:1.
Article50. Liu KK, Dorovini-Zis K. Differential regulation of CD4+ T cell adhesion to cerebral microvascular endothelium by the β-chemokines CCL2 and CCL3. Int J Mol Sci. 2012; 13:16119–40.
Article51. De Laere M, Sousa C, Meena M, Buckinx R, Timmermans JP, Berneman Z, et al. Increased transendothelial transport of CCL3 is insufficient to drive immune cell transmigration through the blood-brain barrier under inflammatory conditions in vitro. Mediators Inflamm. 2017; 2017:6752756.
Article52. Shehabi Y, Howe BD, Bellomo R, Arabi YM, Bailey M, Bass FE, et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. 2019; 380:2506–17.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Delirium in the intensive care unit
- Factors Influencing Intensive Care Unit Nurses’ Competency in Delirium Care in A Tertiary General Hospital
- Investigation of Delirium Occurrence and Intervention Status in Intensive Care Unit at a Hospital and Perception of Delirium by Medical Staff
- Intensive Care Unit Nurse's Knowledge, Nursing Performance, and Stress about Delirium
- Risk Factors for Cognitive Impairment in Intensive Care Unit Survivors