Endocrinol Metab.
2022 Oct;37(5):719-731. 10.3803/EnM.2022.1573.
Independent Skeletal Actions of Pituitary Hormones
- Affiliations
-
- 1The Mount Sinai Bone Program, Departments of Pharmacological Sciences and Medicine, and Center of Translational Medicine and Pharmacology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
- KMID: 2534624
- DOI: http://doi.org/10.3803/EnM.2022.1573
Abstract
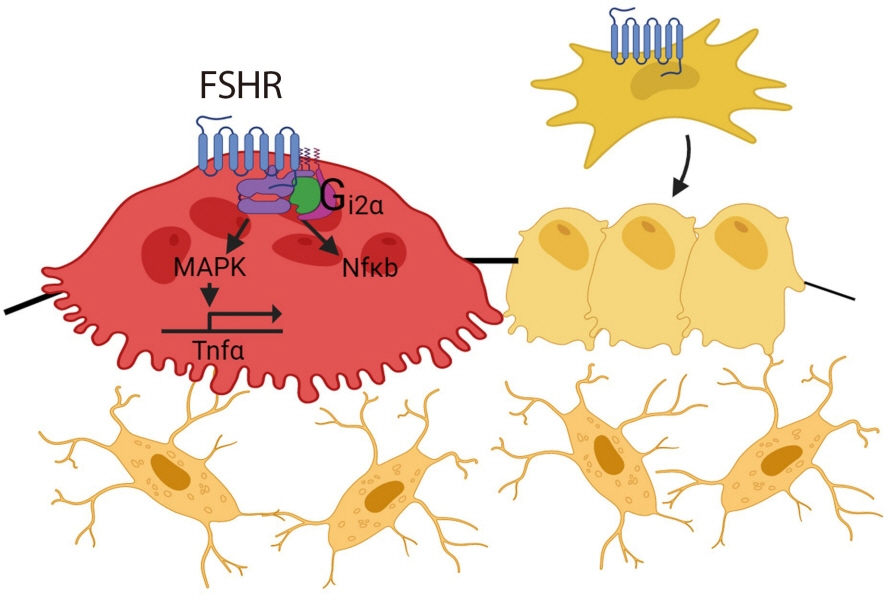
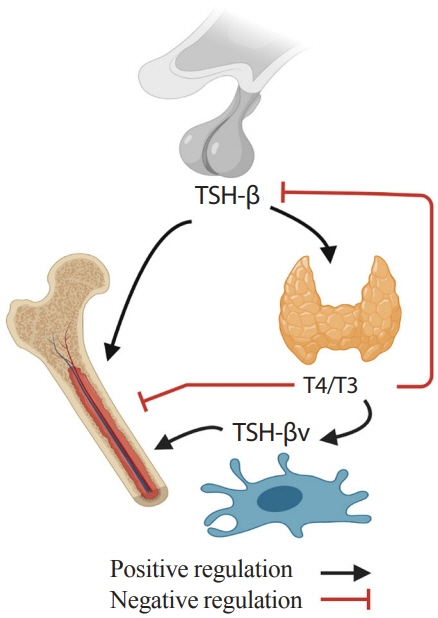
- Over the past years, pituitary hormones and their receptors have been shown to have non-traditional actions that allow them to bypass the hypothalamus-pituitary-effector glands axis. Bone cells—osteoblasts and osteoclasts—express receptors for growth hormone, follicle stimulating hormone (FSH), thyroid stimulating hormone (TSH), adrenocorticotrophic hormone (ACTH), prolactin, oxytocin, and vasopressin. Independent skeletal actions of pituitary hormones on bone have been studied using genetically modified mice with haploinsufficiency and by activating or inactivating the receptors pharmacologically, without altering systemic effector hormone levels. On another front, the discovery of a TSH variant (TSH-βv) in immune cells in the bone marrow and skeletal action of FSHβ through tumor necrosis factor α provides new insights underscoring the integrated physiology of bone-immune-endocrine axis. Here we discuss the interaction of each pituitary hormone with bone and the potential it holds in understanding bone physiology and as a therapeutic target.
Keyword
Figure
Reference
-
1. Davies T, Marians R, Latif R. The TSH receptor reveals itself. J Clin Invest. 2002; 110:161–4.
Article2. Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003; 115:151–62.
Article3. Marians RC, Ng L, Blair HC, Unger P, Graves PN, Davies TF. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptornull mice. Proc Natl Acad Sci U S A. 2002; 99:15776–81.
Article4. Blair HC, Robinson LJ, Sun L, Isales C, Davies TF, Zaidi M. Skeletal receptors for steroid-family regulating glycoprotein hormones: a multilevel, integrated physiological control system. Ann N Y Acad Sci. 2011; 1240:26–31.5. Inoue M, Tawata M, Yokomori N, Endo T, Onaya T. Expression of thyrotropin receptor on clonal osteoblast-like rat osteosarcoma cells. Thyroid. 1998; 8:1059–64.
Article6. Sampath TK, Simic P, Sendak R, Draca N, Bowe AE, O’Brien S, et al. Thyroid-stimulating hormone restores bone volume, microarchitecture, and strength in aged ovariectomized rats. J Bone Miner Res. 2007; 22:849–59.
Article7. Tsai JA, Janson A, Bucht E, Kindmark H, Marcus C, Stark A, et al. Weak evidence of thyrotropin receptors in primary cultures of human osteoblast-like cells. Calcif Tissue Int. 2004; 74:486–91.
Article8. Bassett JH, Williams AJ, Murphy E, Boyde A, Howell PG, Swinhoe R, et al. A lack of thyroid hormones rather than excess thyrotropin causes abnormal skeletal development in hypothyroidism. Mol Endocrinol. 2008; 22:501–12.
Article9. Sun L, Vukicevic S, Baliram R, Yang G, Sendak R, McPherson J, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci U S A. 2008; 105:4289–94.
Article10. Jayaraman A, Kumar TR. Extra-pituitary expressed follicle-stimulating hormone: is it physiologically important? Biol Reprod. 2017; 97:622–6.
Article11. Kumar TR. Extragonadal FSH receptor: is it real? Biol Reprod. 2014; 91:99.12. Zhu D, Li X, Macrae VE, Simoncini T, Fu X. Extragonadal effects of follicle-stimulating hormone on osteoporosis and cardiovascular disease in women during menopausal transition. Trends Endocrinol Metab. 2018; 29:571–80.
Article13. Xiong J, Kang SS, Wang Z, Liu X, Kuo TC, Korkmaz F, et al. FSH blockade improves cognition in mice with Alzheimer’s disease. Nature. 2022; 603:470–6.
Article14. Liu P, Ji Y, Yuen T, Rendina-Ruedy E, DeMambro VE, Dhawan S, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017; 546:107–12.
Article15. Ji Y, Liu P, Yuen T, Haider S, He J, Romero R, et al. Epitope-specific monoclonal antibodies to FSHβ increase bone mass. Proc Natl Acad Sci U S A. 2018; 115:2192–7.
Article16. Stilley JA, Christensen DE, Dahlem KB, Guan R, Santillan DA, England SK, et al. FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta, and the impact of its deletion on pregnancy in mice. Biol Reprod. 2014; 91:74.17. Yee JB, Hutson JC. Biochemical consequences of follicle-stimulating hormone binding to testicular macrophages in culture. Biol Reprod. 1985; 32:872–9.
Article18. Radu A, Pichon C, Camparo P, Antoine M, Allory Y, Couvelard A, et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med. 2010; 363:1621–30.
Article19. Zhang M, Wang Y, Huan Z, Liu Y, Zhang W, Kong D, et al. FSH modulated cartilage ECM metabolism by targeting the PKA/CREB/SOX9 pathway. J Bone Miner Metab. 2021; 39:769–79.
Article20. Robinson LJ, Tourkova I, Wang Y, Sharrow AC, Landau MS, Yaroslavskiy BB, et al. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem Biophys Res Commun. 2010; 394:12–7.
Article21. Cannon JG, Kraj B, Sloan G. Follicle-stimulating hormone promotes RANK expression on human monocytes. Cytokine. 2011; 53:141–4.
Article22. Ulloa-Aguirre A, Zarinan T, Jardon-Valadez E, Gutierrez-Sagal R, Dias JA. Structure-function relationships of the follicle-stimulating hormone receptor. Front Endocrinol (Lausanne). 2018; 9:707.
Article23. Chida D, Nakagawa S, Nagai S, Sagara H, Katsumata H, Imaki T, et al. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc Natl Acad Sci U S A. 2007; 104:18205–10.
Article24. Zhong Q, Sridhar S, Ruan L, Ding KH, Xie D, Insogna K, et al. Multiple melanocortin receptors are expressed in bone cells. Bone. 2005; 36:820–31.
Article25. Blalock JE. Proopiomelanocortin and the immune-neuroendocrine connection. Ann N Y Acad Sci. 1999; 885:161–72.
Article26. Coss D, Yang L, Kuo CB, Xu X, Luben RA, Walker AM. Effects of prolactin on osteoblast alkaline phosphatase and bone formation in the developing rat. Am J Physiol Endocrinol Metab. 2000; 279:E1216–25.
Article27. Seriwatanachai D, Thongchote K, Charoenphandhu N, Pandaranandaka J, Tudpor K, Teerapornpuntakit J, et al. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor kappaB ligand/osteoprotegerin ratio. Bone. 2008; 42:535–46.
Article28. Clement-Lacroix P, Ormandy C, Lepescheux L, Ammann P, Damotte D, Goffin V, et al. Osteoblasts are a new target for prolactin: analysis of bone formation in prolactin receptor knockout mice. Endocrinology. 1999; 140:96–105.29. Sun L, Tamma R, Yuen T, Colaianni G, Ji Y, Cuscito C, et al. Functions of vasopressin and oxytocin in bone mass regulation. Proc Natl Acad Sci U S A. 2016; 113:164–9.
Article30. Li CH, Simpson ME, Evans HM. Isolation of pituitary follicle-stimulating hormone (FSH). Science. 1949; 109:445–6.
Article31. Gemzell CA, Diczfalusy E, Tillinger G. Clinical effect of human pituitary follicle-stimulating hormone (FSH). J Clin Endocrinol Metab. 1958; 18:1333–48.
Article32. Purves HD, Griesbach WE. The site of thyrotrophin and gonadotrophin production in the rat pituitary studied by McManus-Hotchkiss staining for glycoprotein. Endocrinology. 1951; 49:244–64.
Article33. Purves HD, Griesbach WE. The site of follicle stimulating and luteinizing hormone production in the rat pituitary. Endocrinology. 1954; 65:785–93.34. Hisaw CG, Heller EJ, Sevringhaus EL. Does estrogen substitution materially inhibit pituitary gonadotropic potency? Endocrinology. 1942; 30:309–16.
Article35. Byrnes WW, Meyer RK, Finerty JC. Inhibition of gonadotrophic hormone in female parabiotic rats by estrogen and progesterone. Am J Physiol. 1951; 164:26–30.
Article36. Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, et al. FSH directly regulates bone mass. Cell. 2006; 125:247–60.
Article37. Gao J, Tiwari-Pandey R, Samadfam R, Yang Y, Miao D, Karaplis AC, et al. Altered ovarian function affects skeletal homeostasis independent of the action of follicle-stimulating hormone. Endocrinology. 2007; 148:2613–21.
Article38. Morgan I, Coulombe JC, Larsen M, Liu Z, Ferguson VL, Kumar TR. VISIONS: FSH and bone microarchitecture in mice. Mol Reprod Dev. 2022; 89:315.
Article39. Liu S, Cheng Y, Xu W, Bian Z. Protective effects of follicle-stimulating hormone inhibitor on alveolar bone loss resulting from experimental periapical lesions in ovariectomized rats. J Endod. 2010; 36:658–63.
Article40. Zhu LL, Tourkova I, Yuen T, Robinson LJ, Bian Z, Zaidi M, et al. Blocking FSH action attenuates osteoclastogenesis. Biochem Biophys Res Commun. 2012; 422:54–8.
Article41. Gera S, Sant D, Haider S, Korkmaz F, Kuo TC, Mathew M, et al. First-in-class humanized FSH blocking antibody targets bone and fat. Proc Natl Acad Sci U S A. 2020; 117:28971–9.
Article42. Geng W, Yan X, Du H, Cui J, Li L, Chen F. Immunization with FSHβ fusion protein antigen prevents bone loss in a rat ovariectomy-induced osteoporosis model. Biochem Biophys Res Commun. 2013; 434:280–6.
Article43. Zhu LL, Blair H, Cao J, Yuen T, Latif R, Guo L, et al. Blocking antibody to the β-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc Natl Acad Sci U S A. 2012; 109:14574–9.
Article44. Wang J, Zhang W, Yu C, Zhang X, Zhang H, Guan Q, et al. Follicle-stimulating hormone increases the risk of postmenopausal osteoporosis by stimulating osteoclast differentiation. PLoS One. 2015; 10:e0134986.
Article45. Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci U S A. 2006; 103:14925–30.
Article46. Cannon JG, Cortez-Cooper M, Meaders E, Stallings J, Haddow S, Kraj B, et al. Follicle-stimulating hormone, interleukin-1, and bone density in adult women. Am J Physiol Regul Integr Comp Physiol. 2010; 298:R790–8.
Article47. Wu Y, Torchia J, Yao W, Lane NE, Lanier LL, Nakamura MC, et al. Bone microenvironment specific roles of ITAM adapter signaling during bone remodeling induced by acute estrogen-deficiency. PLoS One. 2007; 2:e586.
Article48. Garton M, Martin J, New S, Lee S, Loveridge N, Milne J, et al. Bone mass and metabolism in women aged 45-55. Clin Endocrinol (Oxf). 1996; 44:563–70.
Article49. Steinberg KK, Freni-Titulaer LW, DePuey EG, Miller DT, Sgoutas DS, Coralli CH, et al. Sex steroids and bone density in premenopausal and perimenopausal women. J Clin Endocrinol Metab. 1989; 69:533–9.
Article50. Johnston CC Jr, Hui SL, Witt RM, Appledorn R, Baker RS, Longcope C. Early menopausal changes in bone mass and sex steroids. J Clin Endocrinol Metab. 1985; 61:905–11.
Article51. Ebeling PR, Atley LM, Guthrie JR, Burger HG, Dennerstein L, Hopper JL, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996; 81:3366–71.
Article52. Guthrie JR, Ebeling PR, Hopper JL, Dennerstein L, Wark JD, Burger HG. Bone mineral density and hormone levels in menopausal Australian women. Gynecol Endocrinol. 1996; 10:199–205.
Article53. Peichl P, Griesmacher A, Pointinger P, Marteau R, Hartl W, Gruber W, et al. Association between female sex hormones and biochemical markers of bone turnover in peri- and postmenopausal women. Calcif Tissue Int. 1998; 62:388–94.
Article54. Devleta B, Adem B, Senada S. Hypergonadotropic amenorrhea and bone density: new approach to an old problem. J Bone Miner Metab. 2004; 22:360–4.
Article55. Sowers MR, Greendale GA, Bondarenko I, Finkelstein JS, Cauley JA, Neer RM, et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int. 2003; 14:191–7.
Article56. Veldhuis-Vlug AG, Woods GN, Sigurdsson S, Ewing SK, Le PT, Hue TF, et al. Serum FSH Is associated with BMD, bone marrow adiposity, and body composition in the AGES-Reykjavik study of older adults. J Clin Endocrinol Metab. 2021; 106:e1156–69.
Article57. Xu ZR, Wang AH, Wu XP, Zhang H, Sheng ZF, Wu XY, et al. Relationship of age-related concentrations of serum FSH and LH with bone mineral density, prevalence of osteoporosis in native Chinese women. Clin Chim Acta. 2009; 400:8–13.
Article58. Gallagher CM, Moonga BS, Kovach JS. Cadmium, follicle-stimulating hormone, and effects on bone in women age 42-60 years, NHANES III. Environ Res. 2010; 110:105–11.
Article59. Adami S, Bianchi G, Brandi ML, Giannini S, Ortolani S, DiMunno O, et al. Determinants of bone turnover markers in healthy premenopausal women. Calcif Tissue Int. 2008; 82:341–7.
Article60. Rendina D, Gianfrancesco F, De Filippo G, Merlotti D, Esposito T, Mingione A, et al. FSHR gene polymorphisms influence bone mineral density and bone turnover in postmenopausal women. Eur J Endocrinol. 2010; 163:165–72.
Article61. van der Deure WM, Uitterlinden AG, Hofman A, Rivadeneira F, Pols HA, Peeters RP, et al. Effects of serum TSH and FT4 levels and the TSHR-Asp727Glu polymorphism on bone: the Rotterdam study. Clin Endocrinol (Oxf). 2008; 68:175–81.
Article62. Morris MS. The association between serum thyroid-stimulating hormone in its reference range and bone status in postmenopausal American women. Bone. 2007; 40:1128–34.
Article63. Yang L, Wang H, Guo J, Zheng G, Wei D, Zhang T. Low normal TSH levels and thyroid autoimmunity are associated with an increased risk of osteoporosis in euthyroid postmenopausal women. Endocr Metab Immune Disord Drug Targets. 2021; 21:859–65.
Article64. Noh HM, Park YS, Lee J, Lee W. A cross-sectional study to examine the correlation between serum TSH levels and the osteoporosis of the lumbar spine in healthy women with normal thyroid function. Osteoporos Int. 2015; 26:997–1003.
Article65. Kim DJ, Khang YH, Koh JM, Shong YK, Kim GS. Low normal TSH levels are associated with low bone mineral density in healthy postmenopausal women. Clin Endocrinol (Oxf). 2006; 64:86–90.
Article66. Murphy E, Gluer CC, Reid DM, Felsenberg D, Roux C, Eastell R, et al. Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal women. J Clin Endocrinol Metab. 2010; 95:3173–81.
Article67. Grimnes G, Emaus N, Joakimsen RM, Figenschau Y, Jorde R. The relationship between serum TSH and bone mineral density in men and postmenopausal women: the Tromso study. Thyroid. 2008; 18:1147–55.
Article68. Aubert CE, Floriani C, Bauer DC, da Costa BR, Segna D, Blum MR, et al. Thyroid function tests in the reference range and fracture: individual participant analysis of prospective cohorts. J Clin Endocrinol Metab. 2017; 102:2719–28.
Article69. Bauer DC, Ettinger B, Nevitt MC, Stone KL; Study of Osteoporotic Fractures Research Group. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med. 2001; 134:561–8.
Article70. Abrahamsen B, Jorgensen HL, Laulund AS, Nybo M, Brix TH, Hegedus L. Low serum thyrotropin level and duration of suppression as a predictor of major osteoporotic fractures-the OPENTHYRO register cohort. J Bone Miner Res. 2014; 29:2040–50.
Article71. Blum MR, Bauer DC, Collet TH, Fink HA, Cappola AR, da Costa BR, et al. Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA. 2015; 313:2055–65.72. Segna D, Bauer DC, Feller M, Schneider C, Fink HA, Aubert CE, et al. Association between subclinical thyroid dysfunction and change in bone mineral density in prospective cohorts. J Intern Med. 2018; 283:56–72.
Article73. Kim EH, Jeon YK, Pak K, Kim IJ, Kim SJ, Shin S, et al. Effects of thyrotropin suppression on bone health in menopausal women with total thyroidectomy. J Bone Metab. 2019; 26:31–8.
Article74. Hawkins Carranza F, Guadalix Iglesias S, Luisa De Mingo Dominguez M, Martin-Arriscado Arroba C, Lopez Alvarez B, Allo Miguel G, et al. Trabecular bone deterioration in differentiated thyroid cancer: impact of long-term TSH suppressive therapy. Cancer Med. 2020; 9:5746–55.
Article75. Lee SJ, Kim KM, Lee EY, Song MK, Kang DR, Kim HC, et al. Low normal TSH levels are associated with impaired BMD and hip geometry in the elderly. Aging Dis. 2016; 7:734–43.
Article76. Kim SM, Ryu V, Miyashita S, Korkmaz F, Lizneva D, Gera S, et al. Thyrotropin, hyperthyroidism, and bone mass. J Clin Endocrinol Metab. 2021; 106:e4809–21.
Article77. Martini G, Gennari L, De Paola V, Pilli T, Salvadori S, Merlotti D, et al. The effects of recombinant TSH on bone turnover markers and serum osteoprotegerin and RANKL levels. Thyroid. 2008; 18:455–60.
Article78. Mazziotti G, Sorvillo F, Piscopo M, Cioffi M, Pilla P, Biondi B, et al. Recombinant human TSH modulates in vivo C-telopeptides of type-1 collagen and bone alkaline phosphatase, but not osteoprotegerin production in postmenopausal women monitored for differentiated thyroid carcinoma. J Bone Miner Res. 2005; 20:480–6.
Article79. Karga H, Papaioannou G, Polymeris A, Papamichael K, Karpouza A, Samouilidou E, et al. The effects of recombinant human TSH on bone turnover in patients after thyroidectomy. J Bone Miner Metab. 2010; 28:35–41.
Article80. Sun L, Zhu LL, Lu P, Yuen T, Li J, Ma R, et al. Genetic confirmation for a central role for TNFα in the direct action of thyroid stimulating hormone on the skeleton. Proc Natl Acad Sci U S A. 2013; 110:9891–6.
Article81. Baliram R, Sun L, Cao J, Li J, Latif R, Huber AK, et al. Hyperthyroid-associated osteoporosis is exacerbated by the loss of TSH signaling. J Clin Invest. 2012; 122:3737–41.
Article82. Williams GR, Bassett JH. Thyroid diseases and bone health. J Endocrinol Invest. 2018; 41:99–109.
Article83. Hase H, Ando T, Eldeiry L, Brebene A, Peng Y, Liu L, et al. TNFalpha mediates the skeletal effects of thyroid-stimulating hormone. Proc Natl Acad Sci U S A. 2006; 103:12849–54.84. Diez JJ, Hernanz A, Medina S, Bayon C, Iglesias P. Serum concentrations of tumour necrosis factor-alpha (TNF-alpha) and soluble TNF-alpha receptor p55 in patients with hypothyroidism and hyperthyroidism before and after normalization of thyroid function. Clin Endocrinol (Oxf). 2002; 57:515–21.85. Yamoah K, Brebene A, Baliram R, Inagaki K, Dolios G, Arabi A, et al. High-mobility group box proteins modulate tumor necrosis factor-alpha expression in osteoclastogenesis via a novel deoxyribonucleic acid sequence. Mol Endocrinol. 2008; 22:1141–53.86. Klein JR. Biological impact of the TSHβ splice variant in health and disease. Front Immunol. 2014; 5:155.
Article87. Baliram R, Chow A, Huber AK, Collier L, Ali MR, Morshed SA, et al. Thyroid and bone: macrophage-derived TSH-β splice variant increases murine osteoblastogenesis. Endocrinology. 2013; 154:4919–26.
Article88. Wang HC, Dragoo J, Zhou Q, Klein JR. An intrinsic thyrotropin-mediated pathway of TNF-alpha production by bone marrow cells. Blood. 2003; 101:119–23.89. Liu C, Miao J, Liu X, Zhao Z, Kou T, Liu J, et al. HPT axis-independent TSHβ splice variant regulates the synthesis of thyroid hormone in mice. Mol Med Rep. 2019; 19:4514–22.
Article90. Baliram R, Latif R, Morshed SA, Zaidi M, Davies TF. T3 regulates a human macrophage-derived TSH-β splice variant: implications for human bone biology. Endocrinology. 2016; 157:3658–67.
Article91. van der Poll T, Romijn JA, Wiersinga WM, Sauerwein HP. Tumor necrosis factor: a putative mediator of the sick euthyroid syndrome in man. J Clin Endocrinol Metab. 1990; 71:1567–72.
Article92. Montufar-Solis D, Klein JR. Splenic leukocytes traffic to the thyroid and produce a novel TSHβ isoform during acute listeria monocytogenes infection in mice. PLoS One. 2016; 11:e0146111.
Article93. Ramajayam G, Vignesh RC, Karthikeyan S, Kumar KS, Karthikeyan GD, Veni S, et al. Regulation of insulin-like growth factors and their binding proteins by thyroid stimulating hormone in human osteoblast-like (SaOS2) cells. Mol Cell Biochem. 2012; 368:77–88.
Article94. Baliram R, Latif R, Berkowitz J, Frid S, Colaianni G, Sun L, et al. Thyroid-stimulating hormone induces a Wnt-dependent, feed-forward loop for osteoblastogenesis in embryonic stem cell cultures. Proc Natl Acad Sci U S A. 2011; 108:16277–82.
Article95. Boutin A, Gershengorn MC, Neumann S. β-Arrestin 1 in thyrotropin receptor signaling in bone: studies in osteoblast-like cells. Front Endocrinol (Lausanne). 2020; 11:312.
Article96. Cassier E, Gallay N, Bourquard T, Claeysen S, Bockaert J, Crepieux P, et al. Phosphorylation of β-arrestin2 at Thr383 by MEK underlies β-arrestin-dependent activation of Erk1/2 by GPCRs. Elife. 2017; 6:e23777.
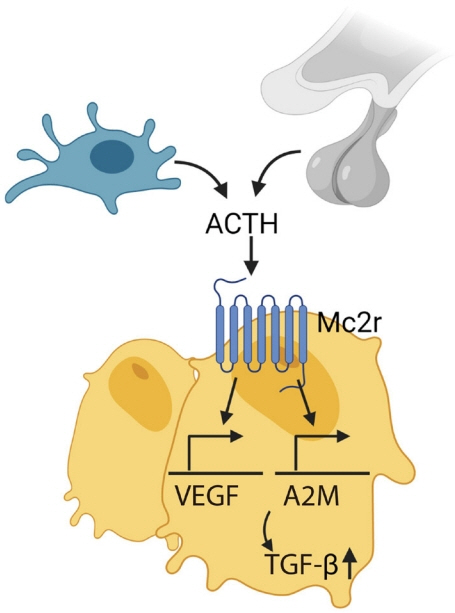
Article97. Zaidi M, Sun L, Robinson LJ, Tourkova IL, Liu L, Wang Y, et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci U S A. 2010; 107:8782–7.
Article98. Tourkova IL, Liu L, Sutjarit N, Larrouture QC, Luo J, Robinson LJ, et al. Adrenocorticotropic hormone and 1,25-dihydroxyvitamin D3 enhance human osteogenesis in vitro by synergistically accelerating the expression of bone-specific genes. Lab Invest. 2017; 97:1072–83.
Article99. Sadeghi F, Vahednia E, Naderi Meshkin H, Kerachian MA. The effect of adrenocorticotropic hormone on alpha-2-macroglobulin in osteoblasts derived from human mesenchymal stem cells. J Cell Mol Med. 2020; 24:4784–90.
Article100. Kerachian MA, Cournoyer D, Harvey EJ, Chow TY, Begin LR, Nahal A, et al. New insights into the pathogenesis of glucocorticoid-induced avascular necrosis: microarray analysis of gene expression in a rat model. Arthritis Res Ther. 2010; 12:R124.
Article101. Minetto M, Reimondo G, Osella G, Ventura M, Angeli A, Terzolo M. Bone loss is more severe in primary adrenal than in pituitary-dependent Cushing’s syndrome. Osteoporos Int. 2004; 15:855–61.
Article102. Sato T, Iwata T, Usui M, Kokabu S, Sugamori Y, Takaku Y, et al. Bone phenotype in melanocortin 2 receptor-deficient mice. Bone Rep. 2020; 13:100713.
Article103. Bouillon R. Growth hormone and bone. Horm Res. 1991; 36 Suppl 1:49–55.
Article104. Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001; 229:141–62.
Article105. Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999; 96:7324–9.
Article106. Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999; 96:7088–92.
Article107. Yakar S, Rosen CJ, Bouxsein ML, Sun H, Mejia W, Kawashima Y, et al. Serum complexes of insulin-like growth factor-1 modulate skeletal integrity and carbohydrate metabolism. FASEB J. 2009; 23:709–19.
Article108. Ohlsson C, Mohan S, Sjogren K, Tivesten A, Isgaard J, Isaksson O, et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009; 30:494–535.
Article109. Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1alpha2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology. 2007; 148:5706–15.110. Wysolmerski JJ. The evolutionary origins of maternal calcium and bone metabolism during lactation. J Mammary Gland Biol Neoplasia. 2002; 7:267–76.111. Sowers M, Eyre D, Hollis BW, Randolph JF, Shapiro B, Jannausch ML, et al. Biochemical markers of bone turnover in lactating and nonlactating postpartum women. J Clin Endocrinol Metab. 1995; 80:2210–6.
Article112. Mantella RC, Vollmer RR, Li X, Amico JA. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003; 144:2291–6.
Article113. Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci U S A. 1996; 93:11699–704.
Article114. Copland JA, Ives KL, Simmons DJ, Soloff MS. Functional oxytocin receptors discovered in human osteoblasts. Endocrinology. 1999; 140:4371–4.
Article115. Colucci S, Colaianni G, Mori G, Grano M, Zallone A. Human osteoclasts express oxytocin receptor. Biochem Biophys Res Commun. 2002; 297:442–5.
Article116. Tamma R, Colaianni G, Zhu LL, DiBenedetto A, Greco G, Montemurro G, et al. Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci U S A. 2009; 106:7149–54.
Article117. Elabd SK, Sabry I, Hassan WB, Nour H, Zaky K. Possible neuroendocrine role for oxytocin in bone remodeling. Endocr Regul. 2007; 41:131–41.118. Sun L, Lizneva D, Ji Y, Colaianni G, Hadelia E, Gumerova A, et al. Oxytocin regulates body composition. Proc Natl Acad Sci U S A. 2019; 116:26808–15.
Article119. Liu X, Shimono K, Zhu LL, Li J, Peng Y, Imam A, et al. Oxytocin deficiency impairs maternal skeletal remodeling. Biochem Biophys Res Commun. 2009; 388:161–6.
Article120. Tamma R, Sun L, Cuscito C, Lu P, Corcelli M, Li J, et al. Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia. Proc Natl Acad Sci U S A. 2013; 110:18644–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pituitary Hormones and Neuropeptides
- The Molecular Pathogenesis of Pituitary Adenomas: An Update
- A Case of Acromegaly Caused by Mixed Gangliocytoma-Adenoma of the Pituitary Gland
- Hypopituitarism Presenting as Osteoporotic Fracture after Cured Tuberculous Meningitis
- Clinical Characteristics and Treatments of Patients with TSH Secreting Pituitary Adenoma