J Stroke.
2022 Sep;24(3):372-382. 10.5853/jos.2022.01046.
Venous Outflow Profiles Are Linked to Clinical Outcomes in Ischemic Stroke Patients with Extensive Baseline Infarct
- Affiliations
-
- 1Department of Diagnostic and Interventional Neuroradiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
- 2Central Institute of Mental Health, Medical Faculty Mannheim, Mannheim, Germany
- 3Department of Neuroradiology, Erasme Medical Center, Brussels, Belgium
- 4Department of Radiology, Jessenius Faculty of Medicine in Martin Clinic of Radiology, Comenius University in Bratislava, Martin, Slovakia
- 5Department of Neurology, Stanford University School of Medicine, Stanford, CA, USA
- 6Department of Diagnostic and Interventional Neuroradiology, University Medical Hospital Basel, Basel, Switzerland
- 7Department of Neuroradiology, MD Andersen Cancer Center, Houston, TX, USA
- 8Department of Radiology, Stanford University School of Medicine, Stanford, CA, USA
- KMID: 2534266
- DOI: http://doi.org/10.5853/jos.2022.01046
Abstract
- Background and Purpose
The benefit of endovascular thrombectomy (EVT) treatment is still unclear in stroke patients presenting with extensive baseline infarct. The use of additional imaging biomarkers could improve clinical outcome prediction and individualized EVT selection in this vulnerable cohort. We hypothesized that cerebral venous outflow (VO) may be associated with functional outcomes in patients with low Alberta Stroke Program Early CT Score (ASPECTS).
Methods
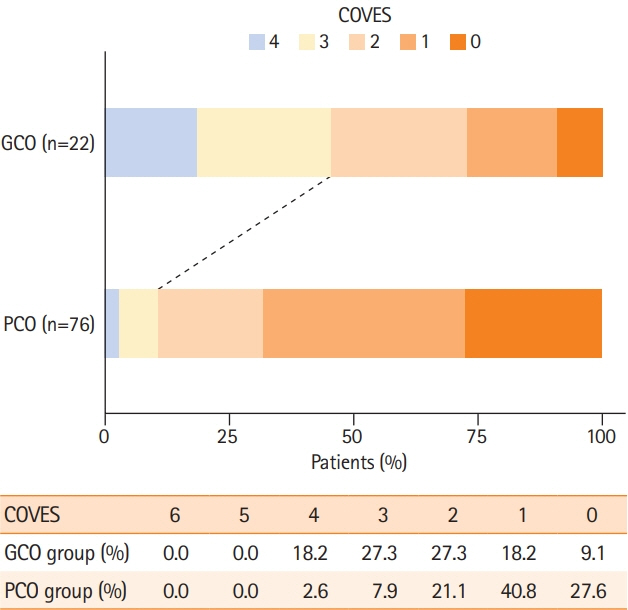
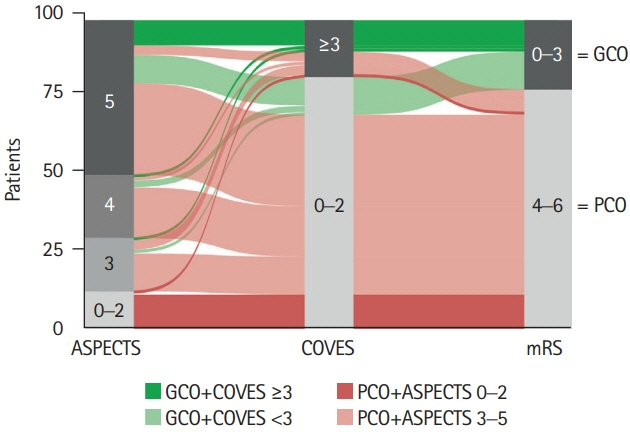
We conducted a retrospective multicenter cohort study of patients with acute ischemic stroke due to large vessel occlusion (AIS-LVO). Extensive baseline infarct was defined by an ASPECTS of ≤5 on admission computed tomography (CT). VO profiles were assessed on admission CT angiography using the Cortical Vein Opacification Score (COVES). Favorable VO was defined as COVES ≥3. Multivariable logistic regression was used to determine the association between cerebral VO and good clinical outcomes (90-day modified Rankin Scale score of ≤3).
Results
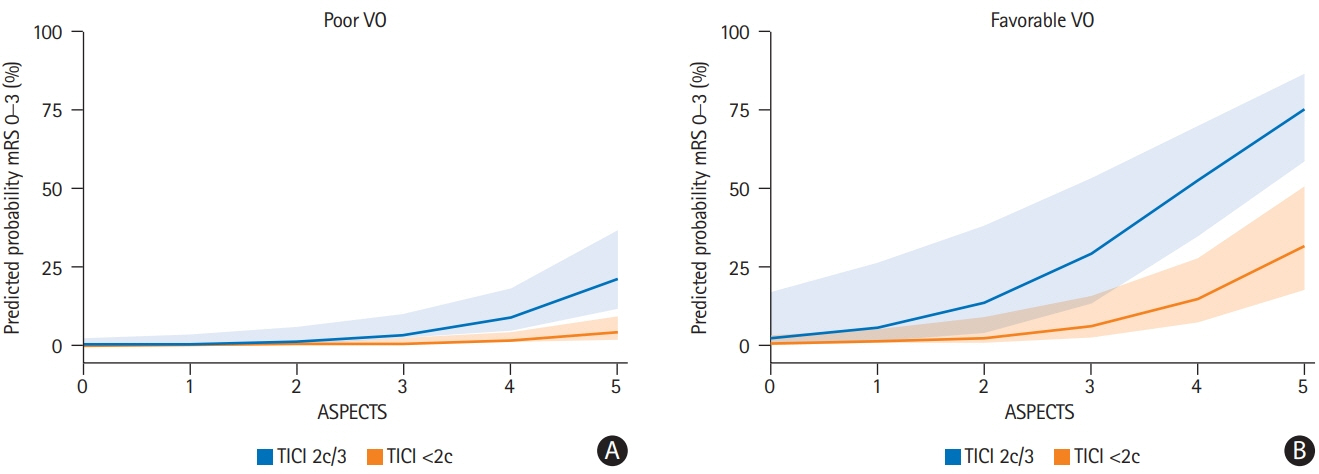
A total of 98 patients met the inclusion criteria. Patients with extensive baseline infarct and favorable VO achieved significantly more often good clinical outcomes compared to patients with unfavorable VO (45.5% vs. 10.5%, P<0.001). Higher COVES were strongly associated with good clinical outcomes (odds ratio, 2.17; 95% confidence interval, 1.15 to 4.57; P=0.024), independent of ASPECTS, National Institutes of Health Stroke Scale, and success of EVT.
Conclusions
Cerebral VO profiles are associated with good clinical outcomes in AIS-LVO patients with extensive baseline infarct. VO profiles could serve as a useful additional imaging biomarker for treatment selection and outcome prediction in low ASPECTS patients.
Figure
Cited by 1 articles
-
Collateral Circulation in Ischemic Stroke: An Updated Review
Gino Maguida, Ashfaq Shuaib
J Stroke. 2023;25(2):179-198. doi: 10.5853/jos.2022.02936.
Reference
-
References
1. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387:1723–1731.2. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018; 378:11–21.3. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018; 378:708–718.4. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–1030.5. Campbell BC, Donnan GA, Lees KR, Hacke W, Khatri P, Hill MD, et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol. 2015; 14:846–854.6. Bendszus M, Bonekamp S, Berge E, Boutitie F, Brouwer P, Gizewski E, et al. A randomized controlled trial to test efficacy and safety of thrombectomy in stroke with extended lesion and extended time window. Int J Stroke. 2019; 14:87–93.7. Yoshimura S, Sakai N, Yamagami H, Uchida K, Beppu M, Toyoda K, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022; 386:1303–1313.8. McMeekin P, White P, James MA, Price CI, Flynn D, Ford GA. Estimating the number of UK stroke patients eligible for endovascular thrombectomy. Eur Stroke J. 2017; 2:319–326.9. Fiehler J, Nawka MT, Meyer L. Persistent challenges in endovascular treatment decision-making for acute ischaemic stroke. Curr Opin Neurol. 2022; 35:18–23.10. Berkhemer OA, Jansen IG, Beumer D, Fransen PS, van den Berg LA, Yoo AJ, et al. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke. 2016; 47:768–776.11. Broocks G, Hanning U, Flottmann F, Schönfeld M, Faizy TD, Sporns P, et al. Clinical benefit of thrombectomy in stroke patients with low ASPECTS is mediated by oedema reduction. Brain. 2019; 142:1399–1407.12. Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009; 40:3001–3005.13. Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010; 41:2316–2322.14. Potreck A, Weyland CS, Seker F, Neuberger U, Herweh C, Hoffmann A, et al. Accuracy and prognostic role of NCCT-ASPECTS depend on time from acute stroke symptom-onset for both human and machine-learning based evaluation. Clin Neuroradiol. 2022; 32:133–140.15. Broocks G, Kniep H, Schramm P, Hanning U, Flottmann F, Faizy T, et al. Patients with low Alberta Stroke Program Early CT Score (ASPECTS) but good collaterals benefit from endovascular recanalization. J Neurointerv Surg. 2020; 12:747–752.16. Faizy TD, Kabiri R, Christensen S, Mlynash M, Kuraitis G, Meyer L, et al. Venous outflow profiles are linked to cerebral edema formation at noncontrast head CT after treatment in acute ischemic stroke regardless of collateral vessel status at CT angiography. Radiology. 2021; 299:682–690.17. Faizy TD, Kabiri R, Christensen S, Mlynash M, Kuraitis GM, Broocks G, et al. Favorable venous outflow profiles correlate with favorable tissue-level collaterals and clinical outcome. Stroke. 2021; 52:1761–1767.18. Hoffman H, Ziechmann R, Swarnkar A, Masoud HE, Gould G. Cortical vein opacification for risk stratification in anterior circulation endovascular thrombectomy. J Stroke Cerebrovasc Dis. 2019; 28:1710–1717.19. Faizy TD, Kabiri R, Christensen S, Mlynash M, Kuraitis G, Mader MM, et al. Association of venous outflow profiles and successful vessel reperfusion after thrombectomy. Neurology. 2021; 96:e2903–e2911.20. van Horn N, Heit JJ, Kabiri R, Mader MM, Christensen S, Mlynash M, et al. Cerebral venous outflow profiles are associated with the first pass effect in endovascular thrombectomy. J Neurointerv Surg. 2021; Nov. 8. [Epub]. https://doi.org/10.1136/neurintsurg-2021-018078.21. Meyer L, Bechstein M, Bester M, Hanning U, Brekenfeld C, Flottmann F, et al. Thrombectomy in extensive stroke may not be beneficial and is associated with increased risk for hemorrhage. Stroke. 2021; 52:3109–3117.22. McDonough R, Elsayed S, Faizy TD, Austein F, Sporns PB, Meyer L, et al. Computed tomography-based triage of extensive baseline infarction: ASPECTS and collaterals versus perfusion imaging for outcome prediction. J Neurointerv Surg. 2021; 13:869–874.23. Pexman JH, Barber PA, Hill MD, Sevick RJ, Demchuk AM, Hudon ME, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001; 22:1534–1542.24. van Horn N, Kniep H, Broocks G, Meyer L, Flottmann F, Bechstein M, et al. ASPECTS interobserver agreement of 100 investigators from the TENSION Study. Clin Neuroradiol. 2021; 31:1093–1100.25. Hair JF, Risher JJ, Sarstedt M, Ringle CM. When to use and how to report the results of PLS-SEM. Eur Bus Rev. 2019; 31:2–4.26. Faizy TD, Kabiri R, Christensen S, Mlynash M, Kuraitis G, Broocks G, et al. Perfusion imaging-based tissue-level collaterals predict ischemic lesion net water uptake in patients with acute ischemic stroke and large vessel occlusion. J Cereb Blood Flow Metab. 2021; 41:2067–2075.27. Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007; 6:258–268.28. Stokum JA, Gerzanich V, Simard JM. Molecular pathophysiology of cerebral edema. J Cereb Blood Flow Metab. 2016; 36:513–538.29. van Horn N, Heit JJ, Kabiri R, Broocks G, Christensen S, Mlynash M, et al. Venous outflow profiles are associated with early edema progression in ischemic stroke. Int J Stroke. 2022; Jan. 5. [Epub]. https://doi.org/10.1177/17474930211065635.30. Broocks G, Meyer L, McDonough R, Bechstein M, Hanning U, Fiehler J, et al. The benefit of thrombectomy in patients with low ASPECTS is a matter of shades of gray-what current trials may have missed. Front Neurol. 2022; 12:718046.31. de Havenon A, Mlynash M, Kim-Tenser MA, Lansberg MG, Leslie-Mazwi T, Christensen S, et al. Results from DEFUSE 3: good collaterals are associated with reduced ischemic core growth but not neurologic outcome. Stroke. 2019; 50:632–638.32. Bhaskar S, Bivard A, Parsons M, Nilsson M, Attia JR, Stanwell P, et al. Delay of late-venous phase cortical vein filling in acute ischemic stroke patients: associations with collateral status. J Cereb Blood Flow Metab. 2017; 37:671–682.33. Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CB, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol. 2018; 17:895–904.34. Sarraj A, Hassan AE, Abraham M, Ribo M, Blackburn S, Chen M, et al. A randomized controlled trial to optimize patient’s selection for endovascular treatment in acute ischemic stroke (SELECT2): study protocol. Int J Stroke. 2022; 17:689–693.35. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European Stroke Organisation (ESO)-European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2019; 11:535–538.36. Jadhav AP, Hacke W, Dippel DW, Simonsen CZ, Costalat V, Fiehler J, et al. Select wisely: the ethical challenge of defining large core with perfusion in the early time window. J Neurointerv Surg. 2021; 13:497–499.37. Ren Z, Huo X, Ma G, Tong X, Kumar J, Pressman E, et al. Selection criteria for large core trials: rationale for the ANGEL-ASPECT study design. J Neurointerv Surg. 2022; 14:107–110.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rat Models for Ischemic Stroke
- Gender Differences in Risk Factor and Clinical Outcome in Patients with Ischemic Stroke
- Effects of sufficient anticoagulation on ischemic stroke outcomes in patients with nonvalvular atrial fibrillation
- Primary neurocritical care involving therapeutic hypothermia for acute ischemic stroke patients with malignant infarct cores
- Antiplatelet Therapy for Secondary Stroke Prevention in Patients with Ischemic Stroke or Transient Ischemic Attack