Korean Circ J.
2022 Oct;52(10):721-736. 10.4070/kcj.2022.0234.
Aortic Stenosis: New Insights in Diagnosis, Treatment, and Prevention
- Affiliations
-
- 1Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA
- KMID: 2534037
- DOI: http://doi.org/10.4070/kcj.2022.0234
Abstract
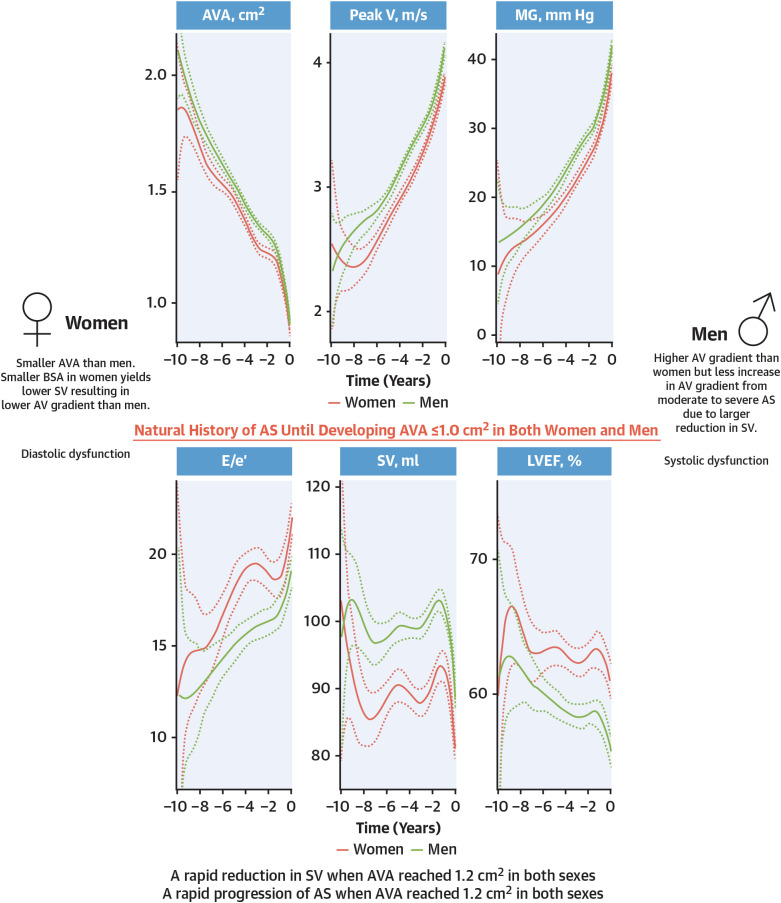
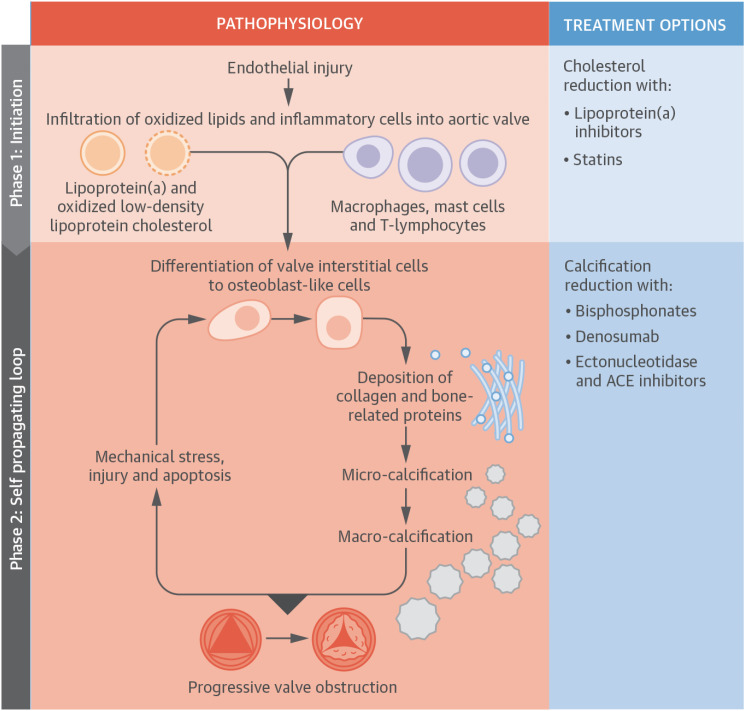
- Aortic stenosis (AS) is one of the most common valvular heart diseases and the number of patients with AS is expected to increase globally as the older population is growing fast. Since the majority of patients are elderly, AS is no longer a simple valvular heart disease of left ventricular outflow obstruction but is accompanied by other cardiac and comorbid conditions. Because of the significant variations of the disease, identifying patients at high risk and even earlier detection of patients with AS before developing symptomatic severe AS is becoming increasingly important. With the proven of efficacy and safety of transcatheter aortic valve replacement (TAVR) in the severe AS population, there is a growing interest in applying TAVR in those with less than severe AS. A medical therapy to reduce or prevent the progression in AS is actively investigated by several randomized control trials. In this review, we will summarize the most recent findings in AS and discuss potential future management strategies of patients with AS.
Figure
Cited by 1 articles
-
Reconsidering the Timing of Aortic Valve Replacement in Symptomatic Normal-Flow Low-Gradient Severe Aortic Stenosis
Hsin-Fu Lee
Korean Circ J. 2023;53(11):756-757. doi: 10.4070/kcj.2023.0183.
Reference
-
1. Bonow RO, Carabello B, de Leon AC Jr, et al. Guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). Circulation. 1998; 98:1949–1984. PMID: 9799219.2. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Society of Cardiovascular Anesthesiologists. Society for Cardiovascular Angiography and Interventions. . ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006; 114:e84–231. PMID: 16880336.3. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017; 38:2739–2791. PMID: 28886619.4. Writing Committee Members. Otto CM, Nishimura RA, et al. ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021; 77:e25–197. PMID: 33342586.5. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014; 129:2440–2492. PMID: 24589852.6. Vahanian A, Beyersdorf F, Praz F, et al. ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022; 43:561–362. PMID: 34453165.7. Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J. 2008; 29:1043–1048. PMID: 18156619.8. Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009; 373:956–966. PMID: 19232707.9. Koide M, Nagatsu M, Zile MR, et al. Premorbid determinants of left ventricular dysfunction in a novel model of gradually induced pressure overload in the adult canine. Circulation. 1997; 95:1601–1610. PMID: 9118531.10. Ito S, Pislaru C, Miranda WR, et al. Left ventricular contractility and wall stress in patients with aortic stenosis with preserved or reduced ejection fraction. JACC Cardiovasc Imaging. 2020; 13:357–369. PMID: 30878438.11. Ward-Smith AJ. Internal Fluid Flow: The Fluid Dynamics of Flow in Pipes and Ducts. New York (NY): Oxford University Press;1980.12. Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997; 95:2262–2270. PMID: 9142003.13. Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005; 111:3290–3295. PMID: 15956131.14. Ito S, Miranda WR, Nkomo VT, Lewis BR, Oh JK. Sex differences in LV remodeling and hemodynamics in aortic stenosis: sex-specific criteria for severe stenosis? JACC Cardiovasc Imaging. 2022; 15:1175–1189. PMID: 35798393.15. Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association scientific statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006; 113:898–918. PMID: 16380542.16. Vriesendorp MD, Groenwold RHH, Herrmann HC, et al. The clinical implications of body surface area as a poor proxy for cardiac output. Struct Heart. 2021; 5:582–587.17. Namasivayam M, He W, Churchill TW, et al. Transvalvular flow rate determines prognostic value of aortic valve area in aortic stenosis. J Am Coll Cardiol. 2020; 75:1758–1769. PMID: 32299587.18. Tobin JR Jr, Rahimtoola SH, Blundell PE, Swan HJ. Percentage of left ventricular stroke work loss. A simple hemodynamic concept for estimation of severity in valvular aortic stenosis. Circulation. 1967; 35:868–879. PMID: 6021776.19. Jander N, Gohlke-Bärwolf C, Bahlmann E, et al. Indexing aortic valve area by body surface area increases the prevalence of severe aortic stenosis. Heart. 2014; 100:28–33. PMID: 23969478.20. Statista Inc. Obesity rate in South Korea 2008–2020 [Internet]. New York (NY): Statista Inc.;2022. cited 2022 Aug 10. Available from: https://www.statista.com/statistics/978106/south-korea-obesity-rate/.21. Rogge BP, Cramariuc D, Lønnebakken MT, et al. Effect of overweight and obesity on cardiovascular events in asymptomatic aortic stenosis: a SEAS substudy (Simvastatin Ezetimibe in Aortic Stenosis). J Am Coll Cardiol. 2013; 62:1683–1690. PMID: 23770175.22. Coisne A, Ninni S, Edmé JL, et al. Obesity paradox in the clinical significance of effective prosthetic orifice area after aortic valve replacement. JACC Cardiovasc Imaging. 2019; 12:208–210. PMID: 29909098.23. National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--the evidence report. Obes Res. 1998; 6(Suppl 2):51S–209S. PMID: 9813653.24. Bogart DB, Murphy BL, Wong BY, Pugh DM, Dunn MI. Progression of aortic stenosis. Chest. 1979; 76:391–396. PMID: 477425.25. Wagner S, Selzer A. Patterns of progression of aortic stenosis: a longitudinal hemodynamic study. Circulation. 1982; 65:709–712. PMID: 7060249.26. Ito S, Miranda WR, Nkomo VT, et al. Reduced left ventricular ejection fraction in patients with aortic stenosis. J Am Coll Cardiol. 2018; 71:1313–1321. PMID: 29566814.27. Strange G, Stewart S, Celermajer D, et al. Poor long-term survival in patients with moderate aortic stenosis. J Am Coll Cardiol. 2019; 74:1851–1863. PMID: 31491546.28. Dahl JS, Eleid MF, Pislaru SV, Scott CG, Connolly HM, Pellikka PA. Development of paradoxical low-flow, low-gradient severe aortic stenosis. Heart. 2015; 101:1015–1023. PMID: 25794516.29. Alcón B, Martínez-Legazpi P, Stewart S, et al. Transvalvular jet velocity, aortic valve area, mortality, and cardiovascular outcomes. Eur Heart J Cardiovasc Imaging. 2022; 23:601–612. PMID: 35137010.30. Ross J Jr, Braunwald E. Aortic stenosis. Circulation. 1968; 38:61–67. PMID: 4894151.31. Park SJ, Enriquez-Sarano M, Chang SA, et al. Hemodynamic patterns for symptomatic presentations of severe aortic stenosis. JACC Cardiovasc Imaging. 2013; 6:137–146. PMID: 23489526.32. Biner S, Rafique AM, Goykhman P, Morrissey RP, Naghi J, Siegel RJ. Prognostic value of E/E′ ratio in patients with unoperated severe aortic stenosis. JACC Cardiovasc Imaging. 2010; 3:899–907. PMID: 20846623.33. Chang SA, Park PW, Sung K, et al. Noninvasive estimate of left ventricular filling pressure correlated with early and midterm postoperative cardiovascular events after isolated aortic valve replacement in patients with severe aortic stenosis. J Thorac Cardiovasc Surg. 2010; 140:1361–1366. PMID: 20381086.34. Dahl JS, Videbæk L, Poulsen MK, et al. Noninvasive assessment of filling pressure and left atrial pressure overload in severe aortic valve stenosis: relation to ventricular remodeling and clinical outcome after aortic valve replacement. J Thorac Cardiovasc Surg. 2011; 142:e77–e83. PMID: 21353251.35. Taniguchi T, Morimoto T, Shiomi H, et al. Prognostic impact of left ventricular ejection fraction in patients with severe aortic stenosis. JACC Cardiovasc Interv. 2018; 11:145–157. PMID: 29289632.36. Dahl JS, Eleid MF, Michelena HI, et al. Effect of left ventricular ejection fraction on postoperative outcome in patients with severe aortic stenosis undergoing aortic valve replacement. Circ Cardiovasc Imaging. 2015; 8:e002917. PMID: 25852129.37. Lancellotti P, Magne J, Dulgheru R, et al. Outcomes of patients with asymptomatic aortic stenosis followed up in heart valve clinics. JAMA Cardiol. 2018; 3:1060–1068. PMID: 30285058.38. Dahl JS, Videbæk L, Poulsen MK, Rudbæk TR, Pellikka PA, Møller JE. Global strain in severe aortic valve stenosis: relation to clinical outcome after aortic valve replacement. Circ Cardiovasc Imaging. 2012; 5:613–620. PMID: 22869821.39. Fries B, Liu D, Gaudron P, et al. Role of global longitudinal strain in the prediction of outcome in patients with severe aortic valve stenosis. Am J Cardiol. 2017; 120:640–647. PMID: 28648391.40. Ng AC, Prihadi EA, Antoni ML, et al. Left ventricular global longitudinal strain is predictive of all-cause mortality independent of aortic stenosis severity and ejection fraction. Eur Heart J Cardiovasc Imaging. 2018; 19:859–867. PMID: 28950306.41. Kusunose K, Goodman A, Parikh R, et al. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging. 2014; 7:938–945. PMID: 25320287.42. Ito S, Miranda WR, Nkomo VT, et al. Prognostic risk stratification of patients with moderate aortic stenosis. J Am Soc Echocardiogr. 2021; 34:248–256. PMID: 33161066.43. Zhu D, Ito S, Miranda WR, et al. Left ventricular global longitudinal strain is associated with long-term outcomes in moderate aortic stenosis. Circ Cardiovasc Imaging. 2020; 13:e009958. PMID: 32268808.44. Lee H, Park JB, Yoon YE, et al. Noncontrast myocardial T1 mapping by cardiac magnetic resonance predicts outcome in patients with aortic stenosis. JACC Cardiovasc Imaging. 2018; 11:974–983. PMID: 29153562.45. Chin CW, Everett RJ, Kwiecinski J, et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging. 2017; 10:1320–1333. PMID: 28017384.46. Weidemann F, Herrmann S, Störk S, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009; 120:577–584. PMID: 19652094.47. Musa TA, Treibel TA, Vassiliou VS, et al. Myocardial scar and mortality in severe aortic stenosis. Circulation. 2018; 138:1935–1947. PMID: 30002099.48. Mentias A, Sarrazin MV, Desai M, Kapadia S, Cram P, Girotra S. Expansion of transcatheter aortic valve replacement in the United States. Am Heart J. 2021; 234:23–30. PMID: 33388288.49. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010; 363:1597–1607. PMID: 20961243.50. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014; 370:1790–1798. PMID: 24678937.51. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011; 364:2187–2198. PMID: 21639811.52. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016; 374:1609–1620. PMID: 27040324.53. Forrest JK, Mangi AA, Popma JJ, et al. Early outcomes with the Evolut PRO repositionable self-expanding transcatheter aortic valve with pericardial wrap. JACC Cardiovasc Interv. 2018; 11:160–168. PMID: 29348010.54. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019; 380:1695–1705. PMID: 30883058.55. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019; 380:1706–1715. PMID: 30883053.56. Carroll JD, Mack MJ, Vemulapalli S, et al. STS-ACC TVT registry of transcatheter aortic valve replacement. J Am Coll Cardiol. 2020; 76:2492–2516. PMID: 33213729.57. Hahn RT, Leipsic J, Douglas PS, et al. Comprehensive echocardiographic assessment of normal transcatheter valve function. JACC Cardiovasc Imaging. 2019; 12:25–34. PMID: 29909110.58. Lee YJ, Lee SJ, Hong SJ, et al. Comparison of transcatheter aortic valve replacement between self-expanding versus balloon-expandable valves in patients with small aortic annulus. Korean Circ J. 2021; 51:222–231. PMID: 33655721.59. Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012; 366:1686–1695. PMID: 22443479.60. Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014; 63:1972–1981. PMID: 24657695.61. Thyregod HG, Steinbrüchel DA, Ihlemann N, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol. 2015; 65:2184–2194. PMID: 25787196.62. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017; 376:1321–1331. PMID: 28304219.63. Grube E, Van Mieghem NM, Bleiziffer S, et al. Clinical outcomes with a repositionable self-expanding transcatheter aortic valve prosthesis: the international FORWARD study. J Am Coll Cardiol. 2017; 70:845–853. PMID: 28797353.64. Manoharan G, Grube E, Van Mieghem NM, et al. Thirty-day clinical outcomes of the Evolut PRO self-expanding transcatheter aortic valve: the international FORWARD PRO study. EuroIntervention. 2020; 16:850–857. PMID: 32748789.65. Bourantas CV, Modolo R, Baumbach A, et al. The evolution of device technology in transcatheter aortic valve implantation. EuroIntervention. 2019; 14:e1826–e1833. PMID: 30719977.66. Tang GH, Zaid S, Michev I, et al. “Cusp-overlap” view simplifies fluoroscopy-guided implantation of self-expanding valve in transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018; 11:1663–1665. PMID: 30139479.67. Yudi MB, Sharma SK, Tang GH, Kini A. Coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement. J Am Coll Cardiol. 2018; 71:1360–1378. PMID: 29566822.68. Rück A, Saleh N, Glaser N. Outcomes following permanent pacemaker implantation after transcatheter aortic valve replacement: SWEDEHEART observational study. JACC Cardiovasc Interv. 2021; 14:2173–2181. PMID: 34620397.69. Auffret V, Puri R, Urena M, et al. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. 2017; 136:1049–1069. PMID: 28893961.70. Van Belle E, Juthier F, Susen S, et al. Postprocedural aortic regurgitation in balloon-expandable and self-expandable transcatheter aortic valve replacement procedures: analysis of predictors and impact on long-term mortality: insights from the FRANCE2 Registry. Circulation. 2014; 129:1415–1427. PMID: 24566199.71. Abdel-Wahab M, Zahn R, Horack M, et al. Aortic regurgitation after transcatheter aortic valve implantation: incidence and early outcome. Results from the German transcatheter aortic valve interventions registry. Heart. 2011; 97:899–906. PMID: 21398694.72. Gotzmann M, Pljakic A, Bojara W, et al. Transcatheter aortic valve implantation in patients with severe symptomatic aortic valve stenosis-predictors of mortality and poor treatment response. Am Heart J. 2011; 162:238–245.e1. PMID: 21835283.73. Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011; 123:299–308. PMID: 21220731.74. Ito S, Miranda WR, Jaffe AS, Oh JK. Prognostic value of N-terminal pro-form B-type natriuretic peptide in patients with moderate aortic stenosis. Am J Cardiol. 2020; 125:1566–1570. PMID: 32204871.75. Moon I, Kim M, Choi JW, et al. Early surgery versus watchful waiting in patients with moderate aortic stenosis and left ventricular systolic dysfunction. Korean Circ J. 2020; 50:791–800. PMID: 32725989.76. Jean G, Van Mieghem NM, Gegenava T, et al. Moderate aortic stenosis in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021; 77:2796–2803. PMID: 34082909.77. Lee JH. Moderate aortic valve stenosis with left ventricular systolic dysfunction: potential role of early aortic valve replacement. Korean Circ J. 2020; 50:801–803. PMID: 32812409.78. Egbe AC, Luis SA, Padang R, Warnes CA. Outcomes in moderate mixed aortic valve disease: Is it time for a paradigm shift? J Am Coll Cardiol. 2016; 67:2321–2329. PMID: 27199054.79. Foroutan F, Guyatt GH, O’Brien K, et al. Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: systematic review of observational studies. BMJ. 2016; 354:i5065. PMID: 27683072.80. Une D, Ruel M, David TE. Twenty-year durability of the aortic Hancock II bioprosthesis in young patients: is it durable enough? Eur J Cardiothorac Surg. 2014; 46:825–830. PMID: 24510909.81. Ueyama H, Kuno T, Takagi H, et al. Meta-analysis comparing valve durability among different transcatheter and surgical aortic valve bioprosthesis. Am J Cardiol. 2021; 158:104–111. PMID: 34465458.82. Pibarot P, Ternacle J, Jaber WA, et al. Structural deterioration of transcatheter versus surgical aortic valve bioprostheses in the PARTNER-2 trial. J Am Coll Cardiol. 2020; 76:1830–1843. PMID: 33059828.83. VARC-3 Writing Committee. Généreux P, Piazza N, et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. 2021; 77:2717–2746. PMID: 33888385.84. Capodanno D, Petronio AS, Prendergast B, et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2017; 38:3382–3390. PMID: 29020344.85. Pawade TA, Newby DE, Dweck MR. Calcification in aortic stenosis: the skeleton key. J Am Coll Cardiol. 2015; 66:561–577. PMID: 26227196.86. Otto CM. Calcific aortic stenosis--time to look more closely at the valve. N Engl J Med. 2008; 359:1395–1398. PMID: 18815402.87. Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994; 90:844–853. PMID: 7519131.88. Nagy E, Eriksson P, Yousry M, et al. Valvular osteoclasts in calcification and aortic valve stenosis severity. Int J Cardiol. 2013; 168:2264–2271. PMID: 23452891.89. Pawade TA, Doris MK, Bing R, et al. Effect of denosumab or alendronic acid on the progression of aortic stenosis: a double-blind randomized controlled trial. Circulation. 2021; 143:2418–2427. PMID: 33913339.90. Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008; 359:1343–1356. PMID: 18765433.91. Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. ASTRONOMER Investigators. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010; 121:306–314. PMID: 20048204.92. Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005; 352:2389–2397. PMID: 15944423.93. Bull S, Loudon M, Francis JM, et al. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor Ramipril In Aortic Stenosis (RIAS trial). Eur Heart J Cardiovasc Imaging. 2015; 16:834–841. PMID: 25796267.94. Bergmark BA, O’Donoghue ML, Murphy SA, et al. An exploratory analysis of proprotein convertase subtilisin/kexin type 9 inhibition and aortic stenosis in the FOURIER trial. JAMA Cardiol. 2020; 5:709–713. PMID: 32347887.95. Choi B, Lee S, Kim SM, et al. Dipeptidyl peptidase-4 induces aortic valve calcification by inhibiting insulin-like growth factor-1 signaling in valvular interstitial cells. Circulation. 2017; 135:1935–1950. PMID: 28179397.96. Lee S, Lee SA, Choi B, et al. Dipeptidyl peptidase-4 inhibition to prevent progression of calcific aortic stenosis. Heart. 2020; 106:1824–1831. PMID: 32917732.97. Kwon JM, Lee SY, Jeon KH, et al. Deep learning-based algorithm for detecting aortic stenosis using electrocardiography. J Am Heart Assoc. 2020; 9:e014717. PMID: 32200712.98. Cohen-Shelly M, Attia ZI, Friedman PA, et al. Electrocardiogram screening for aortic valve stenosis using artificial intelligence. Eur Heart J. 2021; 42:2885–2896. PMID: 33748852.99. Ueda D, Yamamoto A, Ehara S, et al. Artificial intelligence-based detection of aortic stenosis from chest radiographs. Digit Health. 2022; 3:20–28.100. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006; 368:1005–1011. PMID: 16980116.101. Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013; 62:1002–1012. PMID: 23727214.102. Généreux P, Pibarot P, Redfors B, et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J. 2017; 38:3351–3358. PMID: 29020232.103. Kwak S, Lee Y, Ko T, et al. Unsupervised cluster analysis of patients with aortic stenosis reveals distinct population with different phenotypes and outcomes. Circ Cardiovasc Imaging. 2020; 13:e009707. PMID: 32418453.104. Casaclang-Verzosa G, Shrestha S, Khalil MJ, et al. Network tomography for understanding phenotypic presentations in aortic stenosis. JACC Cardiovasc Imaging. 2019; 12:236–248. PMID: 30732719.105. Sengupta PP, Shrestha S, Kagiyama N, et al. A machine-learning framework to identify distinct phenotypes of aortic stenosis severity. JACC Cardiovasc Imaging. 2021; 14:1707–1720. PMID: 34023273.106. Attia ZI, Lerman G, Friedman PA. Deep neural networks learn by using human-selected electrocardiogram features and novel features. Digit Health. 2021; 2:446–455.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Supravalvular Aortic Stenosis
- A Case of Supravalvular and Valvular Aortic Stenosis
- Unicommisural Unicuspid Aortic Valve with Very Severe Aortic Stenosis in a 17-Year-Old Female
- A Case of Severe Aortic Stenosis Patient With High Operative Risk Treated by Transcatheter Aortic-Valve Implantation
- Angiographic analysis of congenital aortic stenosis: study in 20 patients excluding valvular stenosis