Ann Pediatr Endocrinol Metab.
2022 Sep;27(3):214-222. 10.6065/apem.2142184.092.
Clinical and molecular characteristics of infantile-onset diabetes mellitus in Egypt
- Affiliations
-
- 1Faculty of Medicine, Alexandria University, Alexandria, Egypt
- 2University of Exeter Medical School, Institute of Biomedical and Clinical Science, Exeter, UK
- KMID: 2533341
- DOI: http://doi.org/10.6065/apem.2142184.092
Abstract
- Purpose
In patients diagnosed with diabetes mellitus (DM) before the age of 12 months, there is an increasing recognition of diabetes caused by single-gene mutations, also known as monogenic diabetes of infancy or neonatal DM (NDM). This study aimed to classify patients at Alexandria University Children’s Hospital (AUCH) diagnosed with infantile-onset DM into type 1 DM (T1DM) or NDM and to detect differences in molecular characteristics of NDM patients at our center in comparison to other countries.
Methods
This retrospective/prospective observational study was conducted on 39 patients diagnosed with infantile-onset DM (age of onset ≤1 year) at AUCH from January 2003 to November 2020. The patients were divided into 2 groups according to age at the onset of DM: ≤6 months and >6–12 months. Molecular testing was done in patients diagnosed with DM at ≤6 months and those with negative autoantibodies.
Results
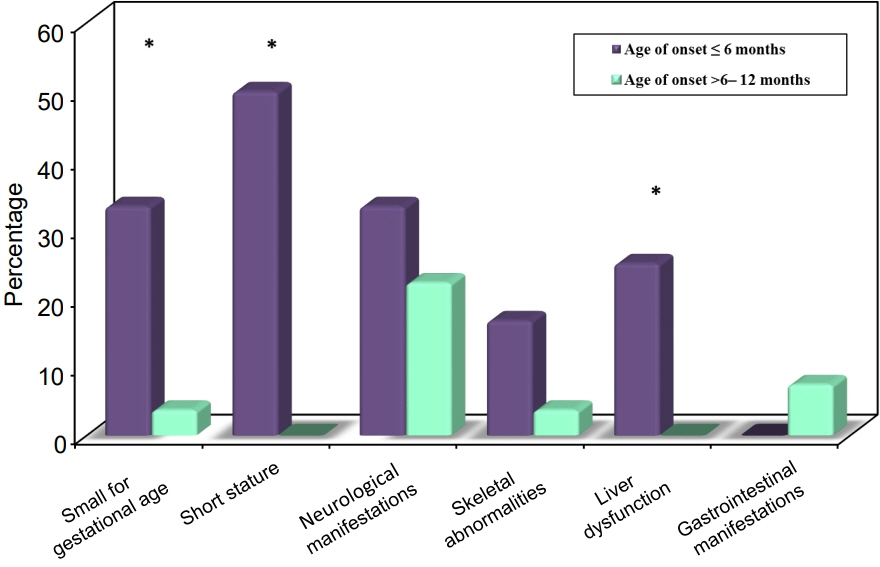
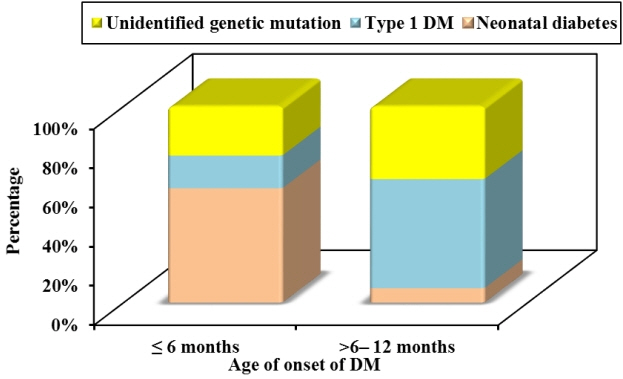
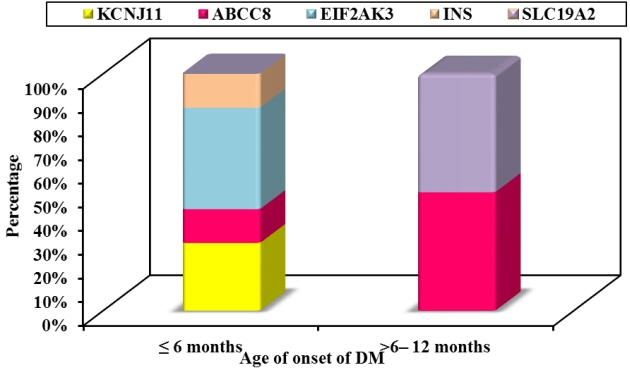
Twelve patients were diagnosed with DM at age ≤6 months and 27 patients were diagnosed between 6–12 months. Seventeen patients (43.6%) had T1DM, whereas 9 patients (23.1%) had genetically confirmed NDM, including 3 harboring novel mutations. The most common genetic causes of NDM were EIF2AK3 mutations (n=3), followed by KCNJ11 (n=2) and ABCC8 (n=2). Other mutations included SLC19A2 (n=1) and INS (n=1). Three patients with potassium ATP channel mutations were transferred from insulin to sulfonylurea treatment.
Conclusion
It is essential to identify patients with NDM clinically and confirm the diagnosis by molecular testing to distinguish them from T1DM as it helps in refining their management, predicting prognosis, and guiding genetic counseling.
Keyword
Figure
Reference
-
References
1. Mayer-Davis EJ, Kahkoska AR, Jefferies C, Dabelea D, Balde N, Gong CX, et al. ISPAD clinical practice consensus guidelines 2018: definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018; 19 Suppl 27(Suppl 27):7–19.2. De Franco E, Flanagan SE, Houghton JA, Lango Allen H, Mackay DJ, Temple IK, et al. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet. 2015; 386:957–63.3. Edghill EL, Flanagan SE, Ellard S. Permanent neonatal diabetes due to activating mutations in ABCC8 and KCNJ11. Rev Endocr Metab Disord. 2010; 11:193–8.4. Ellard S, Lango Allen H, De Franco E, Flanagan SE, Hysenaj G, Colclough K, et al. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia. 2013; 56:1958–63.5. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–24.6. Patel KA, Oram RA, Flanagan SE, De Franco E, Colclough K, Shepherd M, et al. Type 1 diabetes genetic risk score: a novel tool to discriminate monogenic and type 1 diabetes. Diabetes. 2016; 65:2094–9.7. Rubio-Cabezas O, Ellard S. Diabetes mellitus in neonates and infants: genetic heterogeneity, clinical approach to diagnosis, and therapeutic options. Horm Res Paediatr. 2013; 80:137–46.8. Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue KC. The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2009; 10:33–42.9. Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, et al. Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008; 57:1034–42.10. Colombo C, Porzio O, Liu M, Massa O, Vasta M, Salardi S, et al. Seven mutations in the human insulin gene linked to permanent neonatal/infancy-onset diabetes mellitus. J Clin Invest. 2008; 118:2148–56.11. Abujbara MA, Liswi MI, El-Khateeb MS, Flanagan SE, Ellard S, Ajlouni KM. Permanent neonatal diabetes mellitus in Jordan. J Pediatr Endocrinol Metab. 2014; 27:879–83.12. Al-Khawaga S, Mohammed I, Saraswathi S, Haris B, Hasnah R, Saeed A, et al. The clinical and genetic characteristics of permanent neonatal diabetes (PNDM) in the state of Qatar. Mol Genet Genomic Med. 2019; 7:e00753.13. Demirbilek H, Arya VB, Ozbek MN, Houghton JAL, Baran RT, Akar M, et al. Clinical characteristics and molecular genetic analysis of 22 patients with neonatal diabetes from the South-Eastern region of Turkey: predominance of non-KATP channel mutations. Eur J Endocrinol. 2015; 172:697–705.14. Iafusco D, Stazi MA, Cotichini R, Cotellessa M, Martinucci ME, Mazzella M, et al. Permanent diabetes mellitus in the first year of life. Diabetologia. 2002; 45:798–804.15. Johnson MB, Patel KA, De Franco E, Hagopian W, Killian M, McDonald TJ, et al. Type 1 diabetes can present before the age of 6 months and is characterised by autoimmunity and rapid loss of beta cells. Diabetologia. 2020; 63:2605–15.16. Letourneau LR, Carmody D, Wroblewski K, Denson AM, Sanyoura M, Naylor RN, et al. Diabetes presentation in infancy: high risk of diabetic ketoacidosis. Diabetes Care. 2017; 40:e147–8.17. Öngen YD, Eren E, Demirbaş Ö, Sobu E, Ellard S, De Franco E, et al. Genotype and phenotype heterogeneity in neonatal diabetes: a single centre experience in Turkey. J Clin Res Pediatr Endocrinol. 2021; 13:80–7.18. Huopio H, Miettinen PJ, Ilonen J, Nykänen P, Veijola R, Keskinen P, et al. Clinical, genetic, and biochemical characteristics of early-onset diabetes in the Finnish population. J Clin Endocrinol Metab. 2016; 101:3018–26.19. Asl SN, Vakili R, Vakili S, Soheilipour F, Hashemipour M, Ghahramani S, et al. Wolcott-Rallison syndrome in Iran: a common cause of neonatal diabetes. J Pediatr Endocrinol Metab. 2019; 32:607–13.20. Habeb AM, Deeb A, Johnson M, Abdullah M, Abdulrasoul M, Al-Awneh H, et al. Liver disease and other comorbidities in Wolcott-Rallison syndrome: different phenotype and variable associations in a large cohort. Horm Res Paediatr. 2015; 83:190–7.21. Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005; 115:2047–58.22. Bowman P, Sulen Å, Barbetti F, Beltrand J, Svalastoga P, Codner E, et al. Effectiveness and safety of long-term treatment with sulfonylureas in patients with neonatal diabetes due to KCNJ11 mutations: an international cohort study. Lancet Diabetes Endocrinol. 2018; 6:637–46.23. Gloyn AL, Diatloff-Zito C, Edghill EL, Bellanné-Chantelot C, Nivot S, Coutant R, et al. KCNJ11 activating mutations are associated with developmental delay, epilepsy and neonatal diabetes syndrome and other neurological features. Eur J Hum Genet. 2006; 14:824–30.24. Liu M, Sun J, Cui J, Chen W, Guo H, Barbetti F, et al. INSgene mutations: from genetics and beta cell biology to clinical disease. Mol Aspects Med. 2015; 42:3–18.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Study of Genetic Imprinting on 3 Cases of Insulin-Dependent Diabetes Mellitus Developed in Early Infantile Period
- The clinical and immunogenetic characteristics of adult-onset insulin-dependent diabetes mellitus in korea
- Molecular Genetics of Diabetes Mellitus
- Genetic Diseases Associated with Diabetes Mellitus
- Some immunogenetic characteristics and clinical heterogeneity of young adult-onset insulin-dependent diabetes mellitus in Korea