Nutr Res Pract.
2022 Aug;16(4):419-434. 10.4162/nrp.2022.16.4.419.
Ameliorative effect of Abeliophyllum distichum Nakai on benign prostatic hyperplasia in vitro and in vivo
- Affiliations
-
- 1Department of Food Science and Nutrition, Dong-A University, Busan 49315, Korea
- 2Center for Silver-targeted Biomaterials, Brain Busan 21 Plus Program, Dong-A University, Busan 49315, Korea
- 3Division of Food Bioscience, College of Biomedical and Health Sciences, Konkuk University, Chungju 27478, Korea
- 4School of Bio-Science and Food Engineering, Changchun University of Science and Technology, Changchun 130600, China
- 5Department of Microbiology, School of Medicine, Konkuk University, Chungju 27478, Korea
- 6Gyeongnam Agricultural Research and Extension Services, Jinju 52733, Korea
- KMID: 2532093
- DOI: http://doi.org/10.4162/nrp.2022.16.4.419
Abstract
- BACKGROUND/OBJECTIVES
Benign prostatic hyperplasia (BPH) is the most common prostate disease and one of the most common chronic diseases caused by aging in men. On the other hand, there has been no research on BPH using Abeliophyllum distichum Nakai (A.distichum). Therefore, this study investigated the effects of A. distichum on BPH.
MATERIALS/METHODS
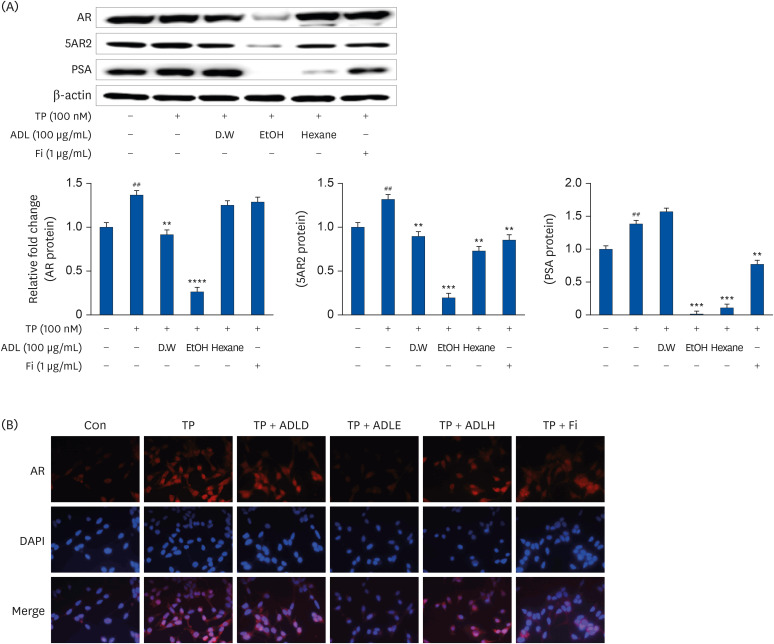
A. distichum leaves were extracted with distilled water, 70% ethanol, and 95% hexane as solvents. Subsequently, the inhibitory effects of each A. distichum extract on androgen receptor (AR) signaling were evaluated in vitro. The testosterone-induced BPH model was then used to confirm the efficacy of A. distichum leaves in 70% ethanol extract (ADLE).
RESULTS
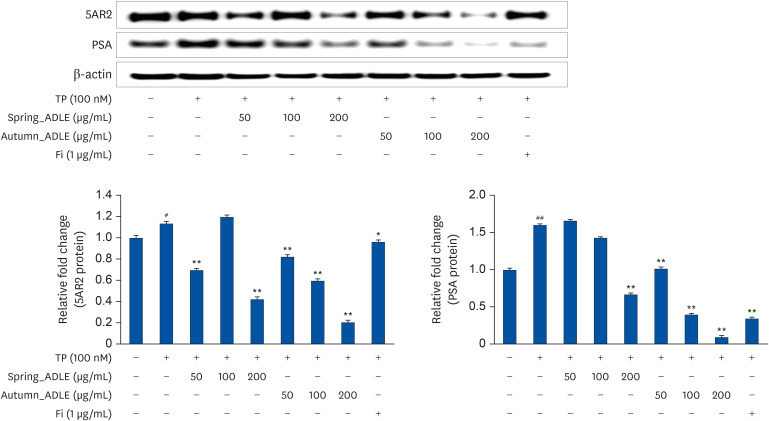
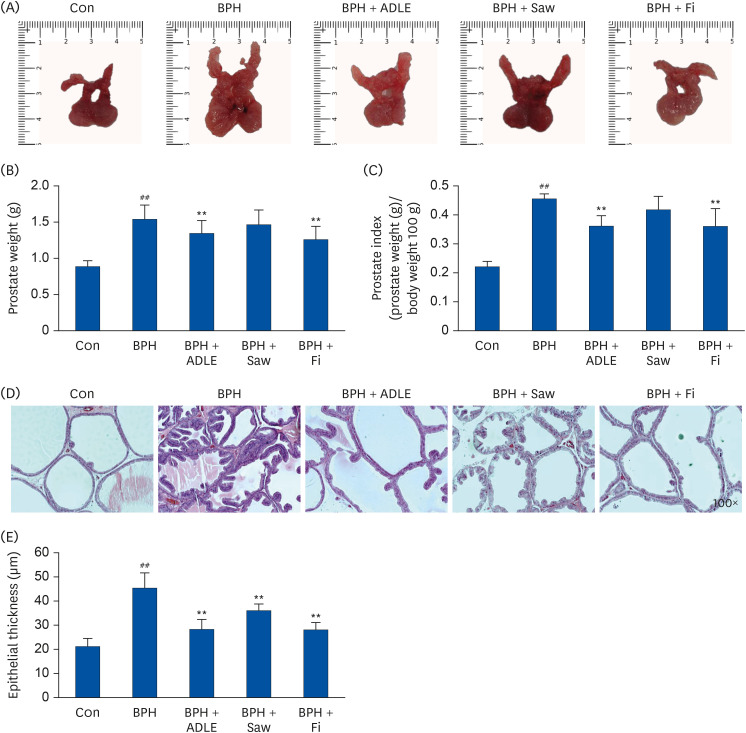
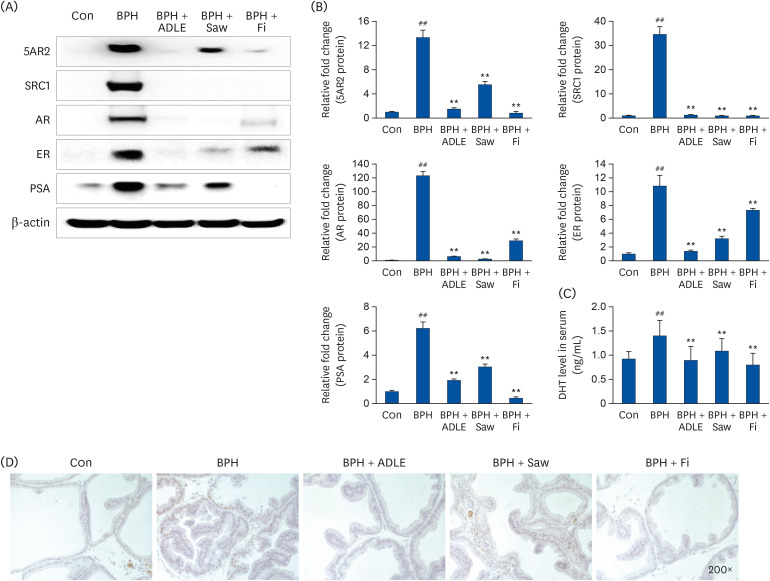
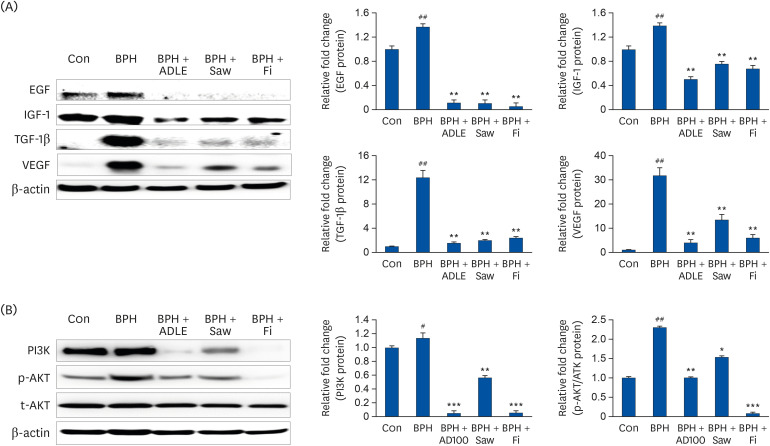
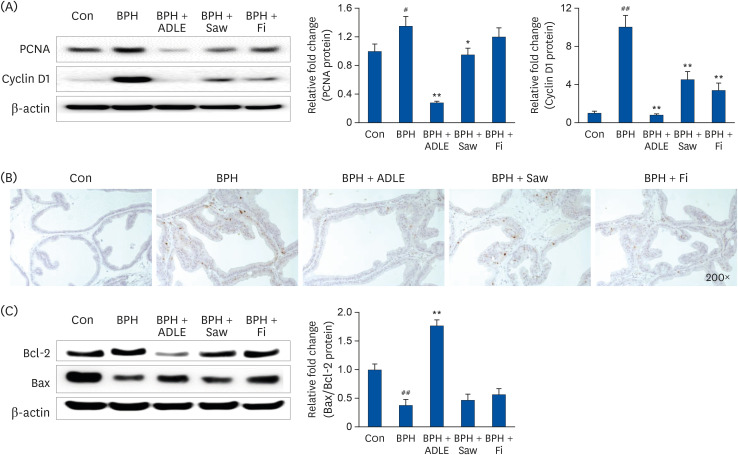
ADLE had the strongest inhibitory effect on AR signaling. A comparison of the activity of ADLE by harvest time showed that the leaves of A. distichum harvested in autumn had a superior inhibitory effect on AR signaling to those harvested at other times. In the BPH rat model, the administration of ADLE reduced the prostate size and prostate epithelial cell thickness significantly and inhibited AR signaling. Subsequently, the administration of ADLE also reduced the expression of growth factors, thereby inactivating the PI3K/AKT pathway.
CONCLUSIONS
An analysis of the efficacy of ADLE to relieve BPH showed that the ethanol extract grown in autumn exhibited the highest inhibitory ability of the androgen-signaling related factors in vitro. ADLE also inhibited the expression of growth factors by inhibiting the expression of the androgen-signaling related factors in vivo. Overall, ADLE is proposed as a functional food that is effective in preventing BPH.
Figure
Reference
-
1. Khoury CK, Bjorkman AD, Dempewolf H, Ramirez-Villegas J, Guarino L, Jarvis A, Rieseberg LH, Struik PC. Increasing homogeneity in global food supplies and the implications for food security. Proc Natl Acad Sci U S A. 2014; 111:4001–4006. PMID: 24591623.
Article2. Sheehy CM, Perry PA, Cromwell SL. Dehydration: biological considerations, age-related changes, and risk factors in older adults. Biol Res Nurs. 1999; 1:30–37. PMID: 11225294.
Article3. Agarwal A, Eryuzlu LN, Cartwright R, Thorlund K, Tammela TL, Guyatt GH, Auvinen A, Tikkinen KA. What is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and women. Eur Urol. 2014; 65:1211–1217. PMID: 24486308.
Article4. Mustonen S, Ala-Houhala IO, Tammela TL. Long-term renal dysfunction in patients with acute urinary retention. Scand J Urol Nephrol. 2001; 35:44–48. PMID: 11291687.
Article5. Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011; 82:184–199. PMID: 21620560.
Article6. Vignozzi L, Rastrelli G, Corona G, Gacci M, Forti G, Maggi M. Benign prostatic hyperplasia: a new metabolic disease? J Endocrinol Invest. 2014; 37:313–322. PMID: 24458832.
Article7. Krieg M, Nass R, Tunn S. Effect of aging on endogenous level of 5 alpha-dihydrotestosterone, testosterone, estradiol, and estrone in epithelium and stroma of normal and hyperplastic human prostate. J Clin Endocrinol Metab. 1993; 77:375–381. PMID: 7688377.
Article8. Andriole G, Bruchovsky N, Chung LW, Matsumoto AM, Rittmaster R, Roehrborn C, Russell D, Tindall D. Dihydrotestosterone and the prostate: the scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J Urol. 2004; 172:1399–1403. PMID: 15371854.
Article9. Luke MC, Coffey DS. Human androgen receptor binding to the androgen response element of prostate specific antigen. J Androl. 1994; 15:41–51. PMID: 7514587.10. Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994; 54:5474–5478. PMID: 7522959.11. Liu RF, Fu G, Li J, Yang YF, Wang XG, Bai PD, Chen YD. Roles of autophagy in androgen-induced benign prostatic hyperplasia in castrated rats. Exp Ther Med. 2018; 15:2703–2710. PMID: 29456672.
Article12. Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993; 7:812–821. PMID: 8491378.
Article13. Waga S, Hannon GJ, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994; 369:574–578. PMID: 7911228.
Article14. Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011; 21:92–101. PMID: 21763611.
Article15. Marzo I, Brenner C, Zamzami N, Jürgensmeier JM, Susin SA, Vieira HL, Prévost MC, Xie Z, Matsuyama S, Reed JC, et al. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998; 281:2027–2031. PMID: 9748162.
Article16. Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc Natl Acad Sci U S A. 1997; 94:3668–3672. PMID: 9108035.
Article17. Pirozzi L, Sountoulides P, Castellan P, Presicce F, Lombardo R, Romero M, De Nunzio C, Tubaro A, Schips L, Cindolo L. Current pharmacological treatment for male LUTS due to BPH: dutasteride or finasteride? Curr Drug Targets. 2015; 16:1165–1171. PMID: 25981606.
Article18. de la Rosette JJ, Alivizatos G, Madersbacher S, Perachino M, Thomas D, Desgrandchamps F, de Wildt M. European Association of Urology. EAU guidelines on benign prostatic hyperplasia (BPH). Eur Urol. 2001; 40:256–263. PMID: 11684840.
Article19. Lepor H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev Urol. 2007; 9:181–190. PMID: 18231614.20. Aggarwal S, Thareja S, Verma A, Bhardwaj TR, Kumar M. An overview on 5α-reductase inhibitors. Steroids. 2010; 75:109–153. PMID: 19879888.
Article21. Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011; 8:872–884. PMID: 21176115.
Article22. Steenkamp V, Gouws MC, Gulumian M, Elgorashi EE, van Staden J. Studies on antibacterial, anti-inflammatory and antioxidant activity of herbal remedies used in the treatment of benign prostatic hyperplasia and prostatitis. J Ethnopharmacol. 2006; 103:71–75. PMID: 16122891.
Article23. Lee TB. Studies on conservation of endemic species Abeliophyllum distichum . J Nat Conserv. 1976; 12:6–10.24. You JH, Lee CH. Analysis on herbaceous communities and flora around Abeliophyllum distichum habitats. Korean J Plant Resour. 2005; 18:315–324.25. Kwon S, Kang H, Kim M, Kim J, Shin H, Kim K. Analysis on the components and safety evaluation of Abeliophyllum distichum Nakai leaves and stems. J Environ Health Sci. 2014; 40:234–244.
Article26. Chang SJ, Jeon NB, Park JW, Jang TW, Jeong JB, Park JH. Antioxidant activities and anti-inflammatory effects of fresh and air-dried Abeliophyllum distichum Nakai leaves. Korean J Food Preserv. 2018; 25:27–35.
Article27. Kim EY, Kim JH, Kim M, Park JH, Sohn Y, Jung HS. Abeliophyllum distichum Nakai alleviates postmenopausal osteoporosis in ovariectomized rats and prevents RANKL-induced osteoclastogenesis in vitro . J Ethnopharmacol. 2020; 257:112828. PMID: 32268206.28. Park GH, Park JH, Eo HJ, Song HM, Woo SH, Kim MK, Lee JW, Lee MH, Lee JR, Koo JS, et al. The induction of activating transcription factor 3 (ATF3) contributes to anti-cancer activity of Abeliophyllum distichum Nakai in human colorectal cancer cells. BMC Complement Altern Med. 2014; 14:487. PMID: 25494848.29. Yoo TK, Kim JS, Hyun TK. Polyphenolic composition and anti-melanoma activity of white forsythia (Abeliophyllum distichum nakai) organ extracts. Plants (Basel). 2020; 9:757.
Article30. Ahn J, Park JH. Effects of Abeliophyllum distichum Nakai flower extracts on antioxidative activities and inhibition of DNA damage. Korean J Plant Res. 2013; 26:355–361.
Article31. Kim HW, Yu AR, Kang M, Sung NY, Lee BS, Park SY, Han IJ, Kim DS, Oh SM, Lee YI, et al. Verbascoside-rich Abeliophyllum distichum Nakai leaf extracts prevent LPS-induced preterm birth through inhibiting the expression of proinflammatory cytokines from macrophages and the cell death of trophoblasts induced by TNF-α. Molecules. 2020; 25:4579.
Article32. Eom J, Thomas SS, Sung NY, Kim DS, Cha YS, Kim KA. Abeliophyllum distichum ameliorates high-fat diet-induced obesity in C57BL/6J mice by upregulating the AMPK pathway. Nutrients. 2020; 12:3320.
Article33. Bent S, Kane C, Shinohara K, Neuhaus J, Hudes ES, Goldberg H, Avins AL. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006; 354:557–566. PMID: 16467543.
Article34. Marks LS, Partin AW, Epstein JI, Tyler VE, Simon I, Macairan ML, Chan TL, Dorey FJ, Garris JB, Veltri RW, et al. Effects of a saw palmetto herbal blend in men with symptomatic benign prostatic hyperplasia. J Urol. 2000; 163:1451–1456. PMID: 10751856.
Article35. Choi YJ, Lee JI, Fan M, Tang Y, Yoon EJ, Ryu YB, Kim EK. Metabolomic analysis of Morus cultivar root extracts and their ameliorative effect on testosterone-induced prostate enlargement in Sprague-Dawley rats. Int J Mol Sci. 2020; 21:1435.
Article36. Cunha GR, Alarid ET, Turner T, Donjacour AA, Boutin EL, Foster BA. Normal and abnormal development of the male urogenital tract. Role of androgens, mesenchymal-epithelial interactions, and growth factors. J Androl. 1992; 13:465–475. PMID: 1293128.37. Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990; 126:1165–1172. PMID: 2298157.
Article38. Ueda T, Mawji NR, Bruchovsky N, Sadar MD. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J Biol Chem. 2002; 277:38087–38094. PMID: 12163482.
Article39. Nandeesha H. Benign prostatic hyperplasia: dietary and metabolic risk factors. Int Urol Nephrol. 2008; 40:649–656. PMID: 18246440.
Article40. McVary KT. A review of combination therapy in patients with benign prostatic hyperplasia. Clin Ther. 2007; 29:387–398. PMID: 17577460.
Article41. Miao MS, Wang ZM, Zhang YL, Gao JL. Effects of total flavonoids from bastard speedwell on benign prostatic hyperplasia rats models. Chin J Mod Appl Pharm. 2011; 28:4–7.42. Dai GC, Hu B, Zhang WF, Peng F, Wang R, Liu ZY, Xue BX, Liu JY, Shan YX. Chemical characterization, anti-benign prostatic hyperplasia effect and subchronic toxicity study of total flavonoid extract of Pteris multifida . Food Chem Toxicol. 2017; 108:524–531. PMID: 27845168.
Article43. Bisson JF, Hidalgo S, Rozan P, Messaoudi M. Therapeutic effect of ACTICOA powder, a cocoa polyphenolic extract, on experimentally induced prostate hyperplasia in Wistar-Unilever rats. J Med Food. 2007; 10:628–635. PMID: 18158833.
Article44. Wu Y, Chhipa RR, Zhang H, Ip C. The antiandrogenic effect of finasteride against a mutant androgen receptor. Cancer Biol Ther. 2011; 11:902–909. PMID: 21386657.
Article45. Carson C 3rd, Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003; 61:2–7.
Article46. Vickman RE, Franco OE, Moline DC, Vander Griend DJ, Thumbikat P, Hayward SW. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: a review. Asian J Urol. 2020; 7:191–202. PMID: 32742923.
Article47. Olaisen C, Müller R, Nedal A, Otterlei M. PCNA-interacting peptides reduce Akt phosphorylation and TLR-mediated cytokine secretion suggesting a role of PCNA in cellular signaling. Cell Signal. 2015; 27:1478–1487. PMID: 25797046.
Article48. Li Z, Shen J, Wu WK, Yu X, Liang J, Qiu G, Liu J. Leptin induces cyclin D1 expression and proliferation of human nucleus pulposus cells via JAK/STAT, PI3K/Akt and MEK/ERK pathways. PLoS One. 2012; 7:e53176. PMID: 23300886.
Article49. Khalaf HA, Jasim RA, Ibrahim IT. Verbascoside—A review of its antitumor activities. Pharmacol Pharm. 2021; 12:109–126.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antioxidant and anti-inflammatory effects and mechanism of Abeliophyllum distichum leaf extract in RAW264.7 macrophages
- A Prominently Large Glans penis as a Possible sign of Benign Prostatic Hyperplasia
- The Effects of Abdominal Obesity on the Increased Prevalence Rate of Hypertension and Diabetes Mellitus in Benign Prostatic Hyperplasia Patients
- The influence of age and endocrine factors on the volume of benign prostatic hyperplasia
- The Extract of Couroupita guianensis Aubl. Ameliorates Benign Prostatic Hyperplasia In Vitro and In Vivo