J Rhinol.
2022 Jul;29(2):82-87. 10.18787/jr.2021.00400.
The Roles of Vascular Endothelial Growth Factor, Angiostatin, and Endostatin in Nasal Polyp Development
- Affiliations
-
- 1Department of Otorhinolaryngology-Head & Neck Surgery, College of Medicine, Inje University, Busan, Republic of Korea
- KMID: 2532009
- DOI: http://doi.org/10.18787/jr.2021.00400
Abstract
- Background and Objectives
Microvascular remodeling and angiogenesis are elements of tissue remodeling characteristic of chronic inflammatory diseases, including nasal polyps (NPs). Angiogenesis reflects the balance between the actions of pro- and anti-angiogenic factors. Many pro-angiogenic factors are known, including vascular endothelial growth factor (VEGF). A number of anti-angiogenic factors (e.g., angiostatin and endostatin) also has been identified. Our objective was to assess the roles of VEGF, angiostatin, and endostatin in NP development.
Methods
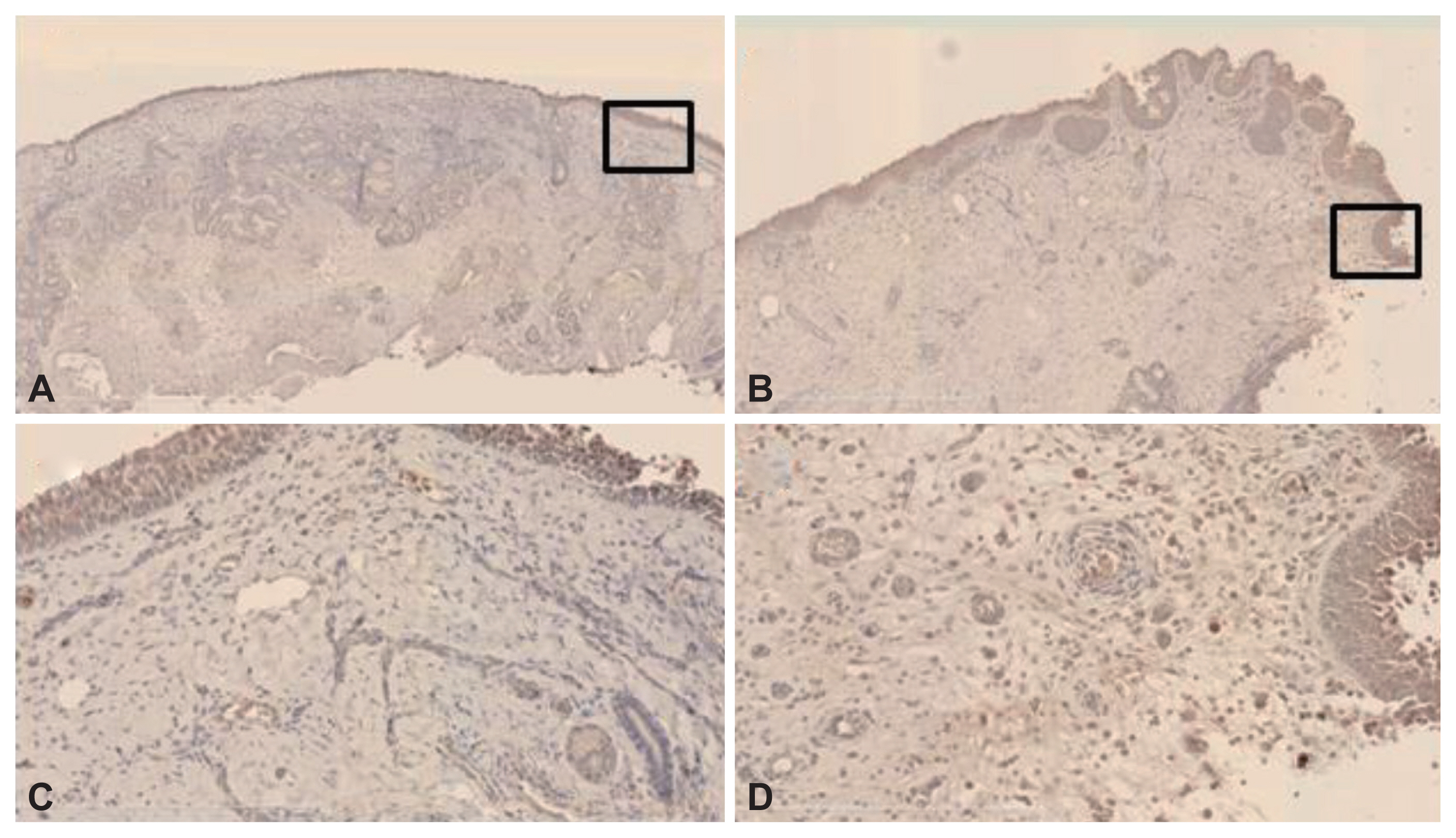
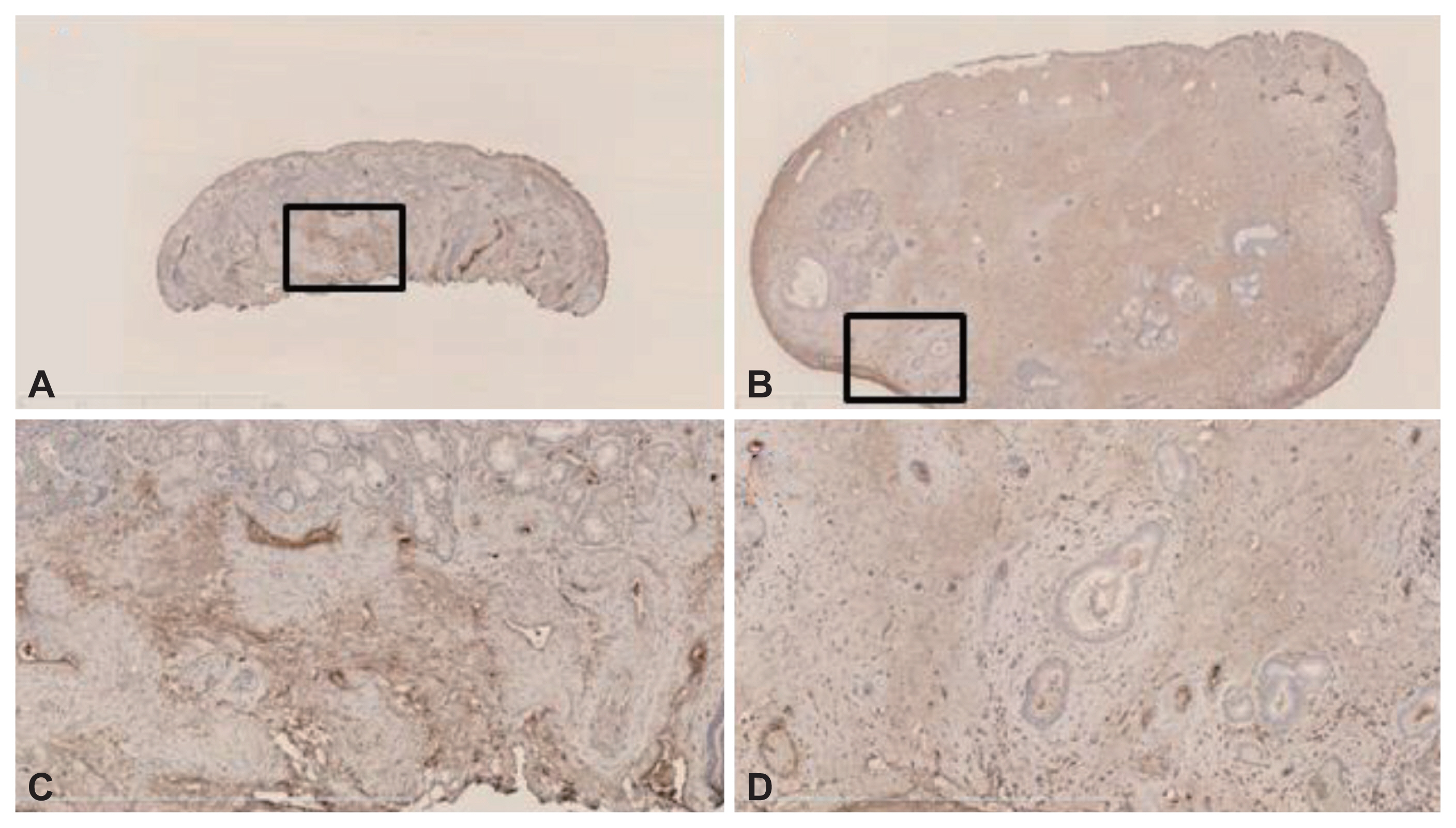
The expression levels of VEGF, angiostatin, and endostatin were measured in NPs harvested during endoscopic endonasal surgery and compared with those in inferior turbinate mucosa (control) samples acquired from patients with hypertrophic rhinitis without allergy. Western blotting and immunohistochemical staining were used to analyze all samples.
Results
The levels of VEGF and angiostatin were significantly higher in the NP subjects than in the controls. Neither the VEGF/angiostatin ratio nor the endostatin level differed significantly between the two groups. However, the VEGF/endostatin ratio was significantly higher in the NP than in the control group. Both the NP and control tissues were diffusely immunoreactive for VEGF, angiostatin, and endostatin.
Conclusion
NP-associated hypoxia can elevate angiostatin level; moreover, an imbalance in the VEGF/endostatin ratio can contribute to NP formation.
Figure
Reference
-
1. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995; 1(1):27–31.2. Ribatti D. Endogenous inhibitors of angiogenesis: a historical review. Leuk Res. 2009; 33(5):638–44.3. Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999; 9(3):211–20.4. Sim BK, MacDonald NJ, Gubish ER. Angiostatin and endostatin: endogenous inhibitors of tumor growth. Cancer Metastasis Rev. 2000; 19(1–2):181–90.5. Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008; 358(19):2039–49.6. Coste A, Brugel L, Maître B, Boussat S, Papon JF, Wingerstmann L, et al. Inflammatory cells as well as epithelial cells in nasal polyps express vascular endothelial growth factor. Eur Respir J. 2000; 15(2):367–72.7. Chen YH, Wu HL, Li C, Huang YH, Chiang CW, Wu MP, et al. Anti-angiogenesis mediated by angiostatin K1-3, K1-4 and K1-4.5. Involvement of p53, FasL, AKT and mRNA deregulation. Thromb Haemost. 2006; 95(4):668–77.8. Moser TL, Stack MS, Asplin I, Enghild JJ, Højrup P, Everitt L, et al. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci U S A. 1999; 96(6):2811–6.9. Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, et al. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci U S A. 2001; 98(12):6656–61.10. Sharma MR, Tuszynski GP, Sharma MC. Angiostatin-induced inhibition of endothelial cell proliferation/apoptosis is associated with the down-regulation of cell cycle regulatory protein cdk5. J Cell Biochem. 2004; 91(2):398–409.11. Troyanovsky B, Levchenko T, Månsson G, Matvijenko O, Holmgren L. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol. 2001; 152(6):1247–54.12. Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M, et al. Endostatin induces endothelial cell apoptosis. J Biol Chem. 1999; 274(17):11721–6.13. Kang HY, Shim D, Kang SS, Chang SI, Kim HY. Protein kinase B inhibits endostatin-induced apoptosis in HUVECs. J Biochem Mol Biol. 2006; 39(1):97–104.14. Kim YM, Hwang S, Kim YM, Pyun BJ, Kim TY, Lee ST, et al. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J Biol Chem. 2002; 277(31):27872–9.15. Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo C, et al. Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci U S A. 2001; 98(3):1024–9.16. Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: developing guidance for clinical trials. Otolaryngol Head Neck Surg. 2006; 135(5 Suppl):S31–80.17. McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001; 164(10 Pt 2):S39–45.18. Hamada N, Kuwano K, Yamada M, Hagimoto N, Hiasa K, Egashira K, et al. Anti-vascular endothelial growth factor gene therapy attenuates lung injury and fibrosis in mice. J Immunol. 2005; 175(2):1224–31.19. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003; 9(6):669–76.20. Claesson-Welsh L, Welsh M, Ito N, Anand-Apte B, Soker S, Zetter B, et al. Angiostatin induces endothelial cell apoptosis and activation of focal adhesion kinase independently of the integrin-binding motif RGD. Proc Natl Acad Sci U S A. 1998; 95(10):5579–83.21. Lucas R, Holmgren L, Garcia I, Jimenez B, Mandriota SJ, Borlat F, et al. Multiple forms of angiostatin induce apoptosis in endothelial cells. Blood. 1998; 92(12):4730–41.22. Redlitz A, Daum G, Sage EH. Angiostatin diminishes activation of the mitogen-activated protein kinases ERK-1 and ERK-2 in human dermal microvascular endothelial cells. J Vasc Res. 1999; 36(1):28–34.23. Pascaud MA, Griscelli F, Raoul W, Marcos E, Opolon P, Raffestin B, et al. Lung overexpression of angiostatin aggravates pulmonary hypertension in chronically hypoxic mice. Am J Respir Cell Mol Biol. 2003; 29(4):449–57.24. Chien CY, Tai CF, Ho KY, Kuo WR, Chai CY, Hsu YC, et al. Expression of hypoxia-inducible factor 1alpha in the nasal polyps by real-time RT-PCR and immunohistochemistry. Otolaryngol Head Neck Surg. 2008; 139(2):206–10.25. O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997; 88(2):277–85.26. Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997; 91(4):443–6.27. Becker CM, Sampson DA, Rupnick MA, Rohan RM, Efstathiou JA, Short SM, et al. Endostatin inhibits the growth of endometriotic lesions but does not affect fertility. Fertil Steril. 2005; 84:Suppl 2. 1144–55.28. Tolstanova G, Deng X, Khomenko T, Garg P, Paunovic B, Chen L, et al. Role of anti-angiogenic factor endostatin in the pathogenesis of experimental ulcerative colitis. Life Sci. 2011; 88(1–2):74–81.29. Hu Y, Hu MM, Shi GL, Han Y, Li BL. Imbalance between vascular endothelial growth factor and endostatin correlates with the prognosis of operable non-small cell lung cancer. Eur J Surg Oncol. 2014; 40(9):1136–42.30. Asai K, Kanazawa H, Otani K, Shiraishi S, Hirata K, Yoshikawa J. Imbalance between vascular endothelial growth factor and endostatin levels in induced sputum from asthmatic subjects. J Allergy Clin Immunol. 2002; 110(4):571–5.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition Effect of Angiostatin and Endostatin on Human Angiogenesis
- Influence of vascular endothelial growth factor (VEGF) and endostatin on coronary artery lesions in Kawasaki disease
- Expression of Vascular Endothelial Growth Factor and Fibronectin in Nasal Polyps: Effects of Topical Corticosteroid
- Expression of Basic Fibroblast Growth Factor(bFGF), Vascular Endothelial Growth Factor(VEGF), and Platelet-Derived Endothelial Cell Growth Factor(PD-ECGF) in Nasal Polyps
- Distribution of Histologic Type of Nasal Polyp and Expression of Vascular Endothelial Growth Factor According to Nasal Polyp Type